Back in February, the Food and Drug Administration (FDA) approved the use of a retinal implant to treat a medical condition known as retinitis pigmentosa.
Recent news on the topic says that, by the end of this year, this so-called bionic eye is to become commercially available in the US.
Furthermore, Second Sight Medical Products, i.e. the company that developed the device, expects that, in the months to come, some 100 retinitis pigmentosa patients will be fitted with this device, CBS News tells us.
People diagnosed with said eye disease experience a gradual and fairly slow degeneration of the photoreceptor cells in their retina. At first, this causes them to lose their night and side vision. In time, their central vision is also affected and, at some point, they go blind altogether.
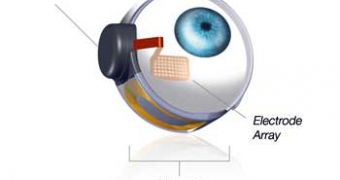
The device, dubbed the Argus II Retinal Prosthesis System, is supposed to partly restore these people's sight by carrying out the tasks that the damaged photoreceptor cells are no longer able to perform.
“The device, which includes a small video camera, transmitter mounted on a pair of eyeglasses, video processing unit (VPU) and an implanted retinal prosthesis (artificial retina), replaces the function of degenerated cells in the retina (a membrane inside the eye) and may improve a patient’s ability to perceive images and movement,” the FDA explained in February.
“The VPU transforms images from the video camera into electronic data that is wirelessly transmitted to the retinal prosthesis,” it further detailed.
The device manufacturers explain that, although the people who will be fitted with it probably won't be able to read fine print or drive a car, the fact remains that they will surely experience a noteworthy improvement in their quality of life.
As Professor Dr. Robert Cykiert with the NYU Langone Medical Center in New York City puts it, “They won't be able to read fine print, but they'll be seeing well enough to possibly walk down the street, avoid bumping into lamp posts and cars, and possibly even cross the street.”
For the time being, one such bionic eye costs about $145,000 (€106,835). The cost is covered by Medicare.
Software updates expected to be released in the not so distant future will help patients see clearer, maybe even in colors.
 14 DAY TRIAL //
14 DAY TRIAL //