Only yesterday, the US Food and Drug Administration made it public news that they had decided to give the green light to the idea of using a retinal implant in order to treat a rare genetic eye disease known to the scientific community as retinitis pigmentosa.
Specialists explain that said medical condition causes people to experience a slow degeneration of the light-sensitive cells in their eye, and that this leads to their losing both side and night vision, and later on to their losing even central vision.
To cut a long story short, those suffering with retinitis pigmentosa are quite likely to go blind at some point in their life, all because of this rare genetic eye disease's continuing to progress.
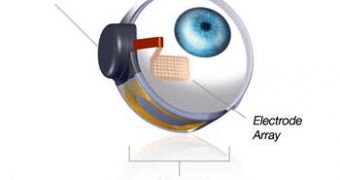
The official website for the US Food and Drug administration explains that the Argus II Retinal Prosthesis System can help people get back some of the eyesight.
As they put it, “The device, which includes a small video camera, transmitter mounted on a pair of eyeglasses, video processing unit (VPU) and an implanted retinal prosthesis (artificial retina), replaces the function of degenerated cells in the retina (a membrane inside the eye) and may improve a patient’s ability to perceive images and movement.”
“The VPU transforms images from the video camera into electronic data that is wirelessly transmitted to the retinal prosthesis,” the US Food and Drug Administration goes on to argue.
This retinal implant is to be made available to adults alone, and those wishing to undergo the medical procedure needed in order to have it installed must be at least 25 years old.
Furthermore, they must be suffering with either a severe, or a profound case of retinitis pigmentosa, they must have an intact inner layer retina function and must be able to prove their past ability to see forms.
“This new surgically implanted assistive device provides an option for patients who have lost their sight to RP – for whom there have been no FDA-approved treatments.”
“The device may help adults with RP who have lost the ability to perceive shapes and movement to be more mobile and to perform day-to-day activities,” commented with respect to this medical procedure Jeffrey Shuren, M.D. and director of the Food and Drug Administration's Center for Devices and Radiological Health.
 14 DAY TRIAL //
14 DAY TRIAL //