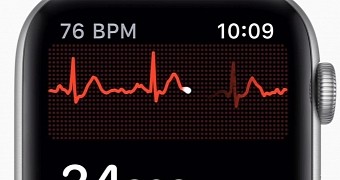
The ECG feature is one of the main highlights of the Apple Watch Series 4, but as it turns out, bringing it to the market is quite a challenge for Apple.
Not only that the company received the FDA approval in the United States literally just a few hours before the September 12 press unveiling, but Apple also needs substantially more time to roll it out in other countries, mostly because of the clearance it must receive in each country.
The United Kingdom is one such example, and a report from 9to5mac revealed that it could take many months before Apple can get ECG ready for the local market.
First and foremost, the Medicines and Healthcare products Regulatory Agency (MHRA) requires companies launching such devices to perform a clinical investigation before the public release, and a pre-study notification is required.
“You may need to carry out a clinical investigation as part of the process to obtain a CE marking for your medical device. You must inform MHRA if you are planning to do this at least 60 days before starting your investigation [providing] some basic details about the investigational device, the intended population, the type of study, and estimated application date,” the official requirements read, according to the said source.
To arrive in the US soon
After the notification is sent, the organization has another 60 days to give its go-ahead. Once the clearance is offered, the company can begin the medical study, which in turn could take up to several months.
Certainly, Apple is well aware of all the steps that it must complete before getting ECG approval in each market, and there’s a chance that the company has already started working with global organizations in this regard.
However, ECG will first and foremost launch in the United States, with a future watchOS update to bring the functionality of the Apple Watch Series 4. No word has been said on the timing, however.
 14 DAY TRIAL //
14 DAY TRIAL //