For more than 600 years, diamonds have been used for a variety of applications, to cut materials, and o polish other diamonds. Now, all these years later, German researchers finally demonstrate how is it that this can be achieved with the hardest material in the world.
Investigators from the Freiburg-based Fraunhofer Institute for Mechanics of Materials IWM, led by Dr. Lars Pastewka and professor Michael Mosele, explain in great details how it's possible for us to use diamonds as tremendously-efficient industrial tools and priceless jewelry.
The new conclusions represent in important breakthrough in the field of science dealing with the analyses of friction and wear, called tribology. The work is published in the latest online issue of the top scientific journal Nature Materials.
In spite of being crucial to a large number of industry branches, tribology has been insufficiently studied, and only a few researchers have dedicated their time to advancing it even further.
Throughout history, diamonds have been machined using cast iron wheels, which were studded with very fine diamond particles. Diamonds can easily scratch other diamonds, craftsmen soon learned.
Experienced diamond grinders soon reached such a high level of efficiency at what they did that they could tell how the cutting was going by listening to the sounds the diamonds made, and by feeling vibrations caused by the wheel during polishing.
One of the main findings of the time was that these carbon compounds react directionally. Chemists later shown that this happens because a diamond is made from lattice planes, which can be cut either easily or with great difficulty, depending on the angle of attack.
Using a newly-developed calculation method, the IWM team was able to answer two main questions – how come diamonds (the roughest materials in the world) can be machined, and why was it a lot easier to machine them at certain inclinations?
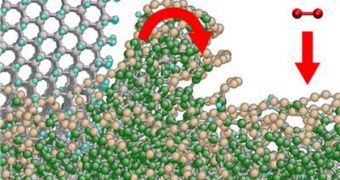
The answer to both questions is mind-numbingly simple. “The moment a diamond is ground, it is no longer a diamond,” says IWM expert Michael Moseler, quoted by AlphaGalileo.
As the diamond-studded wheel spins, a mechanochemical process causes the development of a new glass-like carbon phase on the surface of the diamond being polished.
The crystal orientation (or anisotropy) inside the rocks is what determined how fast the glass-like carbon phase develops and evolves.
“We looked extremely closely at the quantum mechanics of the bonds between the atoms at the surface of the rough diamond breaking. We had to analyze the force field between the atoms in detail,” adds IWM researcher Lars Pastewka.
 14 DAY TRIAL //
14 DAY TRIAL //