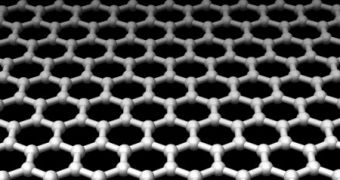
The new material, which was first discovered in 2004, has extremely surprising properties, researchers say, properties that could be put to good use, such as building flexible, transparent electronics, if only scientists were able to synthesize larger quantities of it. Basically, the substance is only one atom thick, and has a hexagonal structure, with carbon atoms arranged neatly in place. It was discovered using graphite, which is the stuff tips of pencils are made of.
Thus far, the only known way to produce graphene was via the use of the "pencil technique," which involves placing a sheet of sticky tape over a pencil and peeling it off afterwards. What remains on the tape are thin layers of graphene. In other words, graphite is made entirely out of graphene, with the difference being that the first substance is made up of tens or hundreds of layers coming from the latter.
Carbon nanotubes, which scientists claim are the future of technology, are also nothing more than graphene sheets rolled up to create a tube. But the overall structure is the same, and the tubes keep all the wonderful properties of the original substance, including mechanical strength, transparency, and even the ability to operate as parts of a processor, working at speeds close to terahertz frequencies.
But mass-introducing these applications in new technological processes is not an easy task, considering that no one has been able to synthesize layers of graphene more than a few centimeters long. Now, a new process promises to make this a lot easier, although it still doesn't offer the key to mass production.
By bombarding a tiny sheet of heated nickel with molecules that contain high amounts of carbon, researchers at the Sungkyunkwan University in Seoul, South Korea, managed to obtain several near-perfect sheets of graphene. Because of the velocity and heat involved in the contact, carbon atoms from the molecules bond with other atoms and create neatly-arranged layers of graphene, which maintain their properties even after the nickel has been peeled away using chemicals.
"Until now, everyone has been using our so-called "pencil technique," but the disadvantage is that the graphite crystals are quite small – it's really painstaking research." The new "technique shows the missing element for the whole story, from finding graphene to making real transistors because it shows that industrial scale production is possible," Andre Geim, the University of Manchester scientist whose team discovered the new compound 5 years ago, told the BBC.
 14 DAY TRIAL //
14 DAY TRIAL //