The 193 nations that signed together the 1989 Montreal Protocol showed, at the time, the level of cooperation that could be achieved at an international level, if the faith of the planet was at stake. Thanks to these nations, chemicals such as chlorofluorocarbons (CFC) and other dangerous substances were entirely phased out, as officials heeded scientists' predictions of what the future might have in store for us if we chose to ignore the problem. Now, experts from NASA’s Goddard Space Flight Center, the Johns Hopkins University, and the Netherlands Environmental Assessment Agency have created a new computer model, showing what “might have been,” if the world hadn't acted.
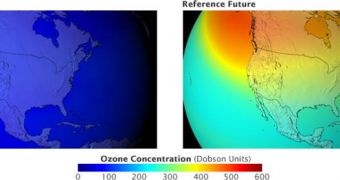
By 2065, which was the reference year for the new simulations, more than 67 percent of the ozone layer surrounding our planet would have been destroyed. The hole above Antarctica would have persisted around the year, and would have had a “twin” at the North Pole. The figures also showed that walking on the streets of mid-latitude cities such as Washington DC or New York for more than five minutes would have been enough to get a sunburn. UV radiation levels, which have the potential to alter the human DNA, would have increased by about 500 percent. With it, cancer rates would have skyrocketed as well, and the effects on plants and animals would have been devastating.
“Ozone science and monitoring have improved over the past two decades, and we have moved to a phase where we [scientists] need to be accountable. We are at the point where we have to ask: Were we right about ozone? Did the regulations work? What kind of world was avoided by phasing out ozone-depleting substances?” Paul Newman, the Goddard scientist who gas led the new study, said. He is also the co-chair of the latest “state of the science” assessment report, which is required by the terms of the Montreal Protocol.
CFC are fairly inactive on the surface of the planet, but, when they reach the upper layers of the atmosphere, they are able to wreak havoc in the ozone layer, by decomposing the molecules that protect our planet from the harmful effects of solar radiation. CFC break into chlorine and bromine under the effects of UV radiation in the higher atmosphere, and these two compounds destroy ozone whenever they come across it. If emissions would have persisted, higher concentrations would have destroyed ozone at an ever-increasing pace.
 14 DAY TRIAL //
14 DAY TRIAL //