Researchers have recently discovered that the earliest complex sugar molecules may have employed the use of silicate ions in order to form even more complex configurations. The new finding could have significant implications for a number of theories on the origins of life, the US team behind the investigation says. It again proposes that the much-maligned formose reaction may have indeed had a very important role in constructing the stepping stone for the complex structures that would later organize themselves in organic molecules, and promote the appearance of life, Chemistry World reports.
It's been more than 60 years since chemists first proposed that the formose reaction must have been important in life's earliest history. This particular chemical interaction basically produces sugars from simple aldehydes. For some reason, many researchers have over the years contested evidence that this reaction may have been involved in the emergence of organic molecules. “Mostly it's been pummeled and attacked because of the fact that the sugars are not stable,. But we don't have anything else. There is no other chemical reaction that works so well, because the formose reaction is so simple,” says Northwestern University expert Joseph Lambert, the leader of the new study.
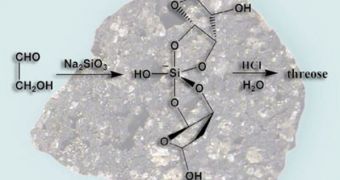
The investigator was inspired in conducting the new research by a scientific paper published in 1991. He became curious in noticing whether sugars would react with sodium silicates, but his investigations took him on a path that eventually revealed the fact that silicate ions also stabilize the sugars, in addition to reacting with them easily. If the results hold up to scrutiny, it could be that the NU team may have found the answer to one of the most exciting questions in chemistry and biology today.
During the investigation, which used mass spectroscopes, the group learned that simple sugars tended to react with each other to form more complex versions of themselves, and then with silicate ions. This lead directly to the production of sugar silicates. The mechanism, the scientists suggest, may have been the same as the one that lead to the formation of the building block of RNA in the eons preceding the development of simple life on Earth. According to University of Stockholm in Sweden prebiotic synthesis expert Nols Holm, the new discovery may very well hold some tremendous merit.
“Their model requires free silicate ions, which you may achieve at high pH. Silicate minerals in rocks – like olivine – are easily weathered, but the secondary minerals that are formed consume most of the silica that is liberated during weathering. However, the process may indeed create high pH,” he says, adding that the reaction would have worked in highly-alkaline environments. “One of the other things that is necessary to explain any sort of role in life is to make it chiral. There are many examples of chiral clays, and so if we can make chiral sugars this way that would be very exciting,” Lambert concludes.
 14 DAY TRIAL //
14 DAY TRIAL //