An international collaboration of researchers managed to identify the structure and mechanisms a protein uses to underly DNA replication. This process is critical to all lifeforms on Earth, experts say.
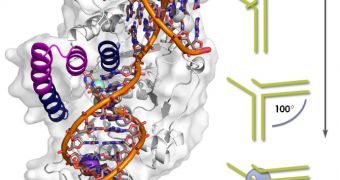
When cells divide, each of the new copies needs to have the exact same genetic material as its precursor did. In charge of ensuring that the genome is properly copied is a series of proteins that include the DNA-slicing flap endonuclease (FEN1).
This molecule plays a critical role in the entire process. As such, learning how it operates was a critical goal in microbiology for many years. Some of the key aspects that distinguish this protein from all the others involved in replication include speed, accuracy, and versatility.
In the new investigation, a collaboration of investigators managed to obtain the entire structure of the FEN1 protein, which also revealed the mechanisms enabling the molecule to act the way it does.
The team was led by experts at the US Department of Energy’s (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) and the Scripps Research Institute (SRI), in La Jolla, California.
“FEN1 has to perform 50 million operations during each replication. It has to do them quickly and it can’t be sloppy,” explains expert John Tainer, who is based at both the Berkeley Lab Life Sciences Division (LSD) and SRI.
“But FEN1 is also important in DNA repair, which presents different challenges. Its ability to target specific repair pathways makes it of urgent interest in cancer research,” the investigator adds.
“We wanted to know how FEN1 can do different jobs, and how the larger family of which it’s a member can perform similar functions on very dissimilar arrangements of DNA,” adds Susan Tsutakawa, the first author of the new paper.
The expert, who is based at LSD, is a part of the team that published the research, entitled “The secret of how FEN1 interacts with DNA lies in its structure,” in the latest issue of the esteemed journal Cell.
“FEN1 is shaped roughly like a left-handed boxing glove,” Tainer explains. He goes on to say that the FEN1 protein binds to unfolded DNA strands at four individual sites across its surface.
In order to conduct the new study, experts used the SIBYLS beamline at the Berkeley Lab Advanced Light Source (ALS). The machine is capable of producing energetic light that can be used to study proteins and similar small molecules.
 14 DAY TRIAL //
14 DAY TRIAL //