In a groundbreaking new study, experts at the Cornell University determined that using single-molecule devices for applications at very small scales iwa both practical and feasible. The group conducted a series of experiments on individual molecules, and learned that these structures could produce the necessary electron flows to power up very small devices. Details of their study are published in the June 11 issue of the top journal Science, the US National Science Foundation (NSF) reports.
The research basically represents one of the first-ever measurements and precision testing of a physical phenomenon known as the underscreened Kondo effect. While relatively inconsequential at large scale, the effects catches own new dimensions when the size of electronic devices is reduced to high miniaturization levels. Given that the current tendency in the industry is to decrease device sizes until they reach the single-molecule limit, the new work could help inform experts in how to ensure the functionality and efficiency of their innovations.
“The main advance in our work is that we show single-molecule devices can be very useful as scientific tools to study an interesting phenomenon that has never before been experimentally accessible,” explains Cornell expert Dan Ralph, who is a professor of physics at the university, and also the leader of the new experiments. “Single-molecule devices can indeed be used as model systems for making detailed quantitative studies of fundamental physics inaccessible by any other technique,” adds Cornell Department of Chemistry and Chemical Biology postdoctoral associate Joshua Parks. He was also the first author of the Science paper.
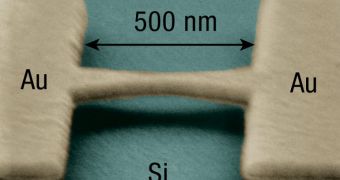
The investigations team, which also included scientists from other countries, used a complex, cobalt-based material, which they cooled down to -273 degrees Fahrenheit, or nearly absolute zero. They were then able to watch the flow of electrons through individual molecules, an accomplishment that has eluded physicists and chemists for decades. The research was funded partially by the NSF Divisions of Materials Research and of Chemistry, as well as by the Cornell Center for Materials Research. The science team also included members from the Institute for Solid State Research and Institute for Advanced Simulation, in Germany and the Bariloche Atomic Center and the Balseiro Institute, in Argentina.
 14 DAY TRIAL //
14 DAY TRIAL //