In some serious heart conditions, patients' only hope for survival is related to receiving a heart 'patch,' a piece of engineered cardiac tissue, which is grafted directly onto their hearts. However, in order for the transplant to survive, it must receive a steady supply of oxygen and nutrients. In an attempt to solve these demands, scientists from the Ben-Gurion University of the Negev, Tel-Aviv University, and the Soroka University Medical Center, in Israel, have developed a way of essentially transforming the human body into a bioreactor, capable of growing working blood vessels in a bioengineered cardiac patch.
Their attempts, which are detailed in the latest issue of the journal Proceedings of the National Academy of Sciences (PNAS), may pave the way for other research groups to create the first bioengineered materials capable of identifying, sticking to, and healing damaged heart tissue. The issue is that, first, they need a scaffold for the new cardiac patch. Onto this scaffold, new cells must then be grafted and grown, which is done in an external bioreactor.
Two main types of cells could be placed on these 3D scaffoldings, the team reports. Most importantly, stem cells that have the ability to differentiate into any type of cardiac cells are needed, and then muscle cells, which could directly benefit the pericardium, by improving its contractions, and thus the heart's pumping abilities. “What they haven't generally focused on is strategies to create the infrastructure to support these myocytes,” Harvard Medical School and Brigham and Women's Hospital Professor of Health Sciences and Technology Frederick Schoen argues.
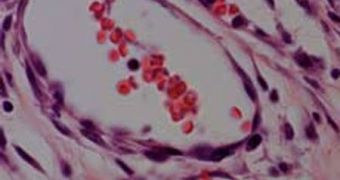
It is absolutely essential that the scaffolds and their cells, regardless of the type, be drenched in blood. In healthy tissue, each of the heart cells is fueled with oxygen and nutrients by two capillaries, which is a set-up that must be exactly mimicked. Otherwise, we end up with an M&M-like structure – dead cells on the inside, and live cells on the outside, Columbia University Professor of Biomedical Engineering Gordana Vunjak-Novakovic explains, quoted by Technology Review.
In their experiments, the Israeli team members tried a new approach. They seeded scaffolds laden with cellular and blood vessel growth factors in the omentum, the membrane that supports the abdominal organs, which is naturally very richly irrigated. After a week, when the scaffolds were populated with mature cells, they were extracted and placed on the animals' hearts. One month later, the patches had not only survived, but they integrated seamlessly into the hearts of the happy mice. The organs were also improved by the patches and exhibited more muscle fibers that contracted more powerfully than before.
 14 DAY TRIAL //
14 DAY TRIAL //