According to a paper published in the scientific journal Nature Chemistry this past September 15, evidence at hand indicates that buckyballs form when larger structures break down.
Thus, contrary to previous theories, they are not constructed atom by atom, much as a kid would build a Lego model.
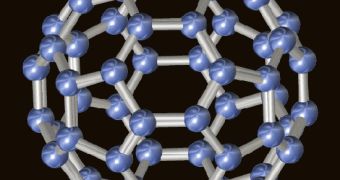
Scientists working with the Virginia Tech Carilion Research Institute explain that buckyballs, whose “official” name is fullerenes, are basically carbon molecules whose shape resembles that of a soccer ball.
They are known to form when asteroids collide with our planet, and their presence has also been documented in interstellar space.
Investigations carried out thus far have shown that they could be used in the fields of medicine, solar energy and optoelectronics, EurekAlert tells us.
However, because the scientific community is yet unaware of how it is exactly that they come into being, it has been fairly difficult to effectively use them on a large scale.
Scientist Harry Dorn and his fellow researchers now say that, according to their investigations, chances are that fullerenes start their life as graphene, i.e. a sheet of carbon measuring just one atom in thickness.
Specifically, they come into being when certain molecular bonds in the sheet of carbon break down.
Though this process initially leads to the formation of asymmetrical fullerenes, researcher Harry Dorn argues that these molecules can alter their shape, and become stable and symmetrical fullerenes.
The scientists explain that, because they are not much different to cages, fullerenes can serve to carry various compounds to certain areas in the human body.
Thus, ongoing experiments are trying to determine whether or not these carbon cages could serve to transport therapeutic radioactive ions to cancer cells and tissues.
What's more, fullerenes carrying gadolinium have been documented to be 40% more effective as a contrast agent for MRI scans than the compounds presently used for this purpose.
Specialists say that, because a fullerene's load is locked away inside it and not allowed to make contact with the surrounding environment, the risk for unwanted side effects is also considerably reduced.
“Understanding the molecular mechanics of how fullerenes and their many variations are formed is not just a curiosity. It would give us insights into new, better ways to prepare them,” Harry Dorn argues.
“Fullerenes and metallofullerenes are already involved in hundreds of biomedical studies. The ability to create large numbers of a wide variety of metallofullerenes [i.e. fullerenes carrying atoms of metal inside them] would be a giant building block that would take the field to new heights,” he adds.
 14 DAY TRIAL //
14 DAY TRIAL //