Some time has passed since fuel cells first appeared, however they are still facing serious problems related to hydrogen to electricity conversion efficiency and the cost of materials used in their construction. Brown professor of chemistry, Shouheng Sun, believes that he has found a solution to boosting the efficiency of fuel cells in shaping platinum into a cubical form. Platinum is routinely used in fuel cell designs as catalyzer in the reaction of oxygen reduction.
By implying the use of platinum, the energy required to start the oxygen reduction reaction is reduced. In the presence of platinum electrons are stripped from the hydrogen atoms and joined with oxygen atoms. The flow of electrons through the fuel cell membrane determines an electrical current which can be harnessed and used to provide with power for a certain electric device. The only byproduct of the hydrogen-oxygen redox reaction is water, thus electric energy produced with fuel cells is non-polluting, probably the most important and only factor which is still driving the research into fuel cells.
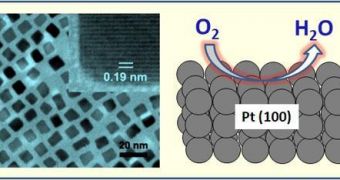
The problem is that platinum is the best catalyzer for this kind reaction and the overall efficiency so far is very low in relation to the true potential of a redox reaction involving hydrogen and oxygen. Scientific efficiency maximization can only be achieved by increasing the surface area and optimizing the shape of the platinum catalyzer, thus in his study, Sun created a nanoscale cube-shaped platinum catalyzer guaranteed to enhance the efficiency of energy conversion.
"For the first time, we can control the morphology of the particle to make it more like a cube. People have had very limited control over this process before. Now we have shown it can be done uniformly and consistently," said professor Sun.
With the help of graduate engineer student Chao Wang and engineers from Hitachi Maxwell Ltd., Sun was able to create platinum acetylacetonate with traces of iron pentacarbonyl in polyhedron and cube shapes in different sizes, depending on the temperature range. The design not only enhances the efficiency of the reaction, but it is also resistant to sulfate absorption in the fuel cell solution.
"For this reaction, the shape is more important than the size," said Sun. Although the catalyzer has not been tested yet, in combination with a polymer electrolyte membrane, it should provide with a higher conversion efficiency than any previous design. "It's like science fiction, but we're a step closer now to the reality of developing a very efficient platinum catalyst for hydrogen cars than produce only water as exhaust," Sun said.
 14 DAY TRIAL //
14 DAY TRIAL //