Ever since American thinker Benjamin Franklin and French physicist Charles-Augustin de Coulomb laid the groundwork for this theory, it has become axiomatic to say that opposite charges attract. This has been proven countless times in a wide array of experiments, and numerous industrial processes are currently based on the knowledge that two particles of the same substance, sporting opposite charges, always attract. However, a new scientific investigation puts a dent in the theory, showing that, if, for instance, droplets of a substance are too charged, then they repel each other, ScienceNow reports.
The find was made by accident in 2005, by University of California in Davis (UCD) chemical engineer William Ristenpart, who was trying to study the effects of electric charges on water droplets, suspended in an oily solution. Usually, in such a setup, when two oppositely charged droplets meet, they exert a mutual attraction force on each other. But, when the expert turned on the electric charge at a higher level, he noticed that the droplets started repelling each other, rather than attracting. “I thought it was fascinating and very confusing,” Ristenpart says.
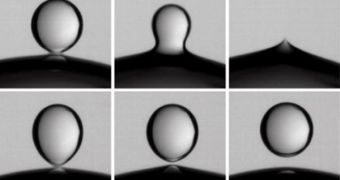
In the case of average droplets meeting and attracting, a series of phenomena occurs. First, because the droplets are flexible, they deform when they draw close to each other, a process that gives birth to structures known as Taylor cones on their surfaces. These cones eventually merge, and then all of the droplets are pulled together. Over the past three years, Ristenpart and colleagues Andrew Belmonte, Jacy Bird, and Howard Stone have spent their time at the Harvard University, trying to find out what causes the abnormal behavior when the electrical charge is too high.
They determined that, in instances where low or medium charges were used, the droplets tended to form short and wide Taylor cones. When higher charges were applied, the attraction force was so great, that the cones became long and thin, forming an angle at their tips. This difference causes the weird behavior, the team writes in the September 17th issue of the journal Nature.
When the bridge between the droplets is short and wide, the surface tension of the liquid essentially pulls the two structures together. When the bridge is long, thin and angled, other forces in the individual take over, and pull the liquid back on itself, thus generating the rejection phenomenon. The find could have vast implications in many fields, ranging from how to perform mass spectrometry, to producing better colors and explaining how rain forms.
 14 DAY TRIAL //
14 DAY TRIAL //