A science group at the University of Washington has recently developed a new system of growing stem cells in the laboratory, without the risk of contamination. Their scaffold eliminates the possibility of these cells being invaded by animal byproducts, as was often the case in various studies. In fact, it was recently discovered that all embryonic stem cells (ESC) that scientists used in 2005 were contaminated, from the medium that was used in Petri dishes to grow them. Animal-based “feeder layers” are still widely used, and the new scaffold is designed specifically to eliminate them, ScienceDaily reports.
The finding about the 2005 ESC research cell lines was very worrying. If those cells had been implanted in patients during therapy, then there would have been a great chance that these people's immune systems rejected the transplants. But the new approach ensures that this never happens. It features a three-dimensional structure, which accurately mimics the binding sites stem cells regularly use. This allows for them to grow in an environment that is clean, and also bio-degradable, a great asset when it comes to constructing more complex organs for transplantation.
In a paper accompanying the finding, which appears in the latest issue of the respected scientific journal Biomaterials, UW researchers underline that ESC grow readily on this type of scaffold, at the same rate that takes place in regular Petri dishes featuring feeder layers. “The major challenge for stem cell therapy today is it's very difficult to make a lot of them with high purity. So far it seems like this material is very good for stem cell renewal,” UW Professor of Materials Science and Engineering Miqin Zhang, who is also the lead author of the journal entry, says.
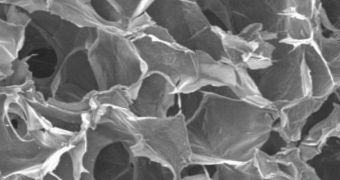
In addition to the purity advantage, the scaffold also allows the cells to grow in an environment that replicates the one inside the human body. In Petri dishes, they grow in very thin layers, whereas in the new device they form in more complex, 3D structures. “Three-dimensional scaffolds are an active area of research. They are not commonly used yet, but will be important to transition embryonic stem cells to the clinic. To date, nobody has found a perfect matrix,” UW Professor of Comparative Medicine Carol Ware, who is also a stem-cell expert, adds.
The scaffold is cylindrical in shape, and is entirely made up of the crustacean shell chemical chitosan, and alginate, a gelatinous compound that is readily found in algae. The abundance of raw materials that are required by this support structure could mean that cheap mass production will be possible. “This scaffold mimics the extracellular matrix at the atomic level, and so the cells are able to grow in this environment,” Zhang concludes.
 14 DAY TRIAL //
14 DAY TRIAL //