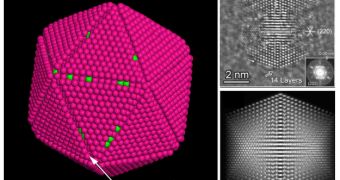
A new form of platinum has been produced by a research team composed of electrochemists and materials scientists, which can greatly increase the present catalytic chemical processes such as those used to catalyze fuel oxidation and produce hydrogen for fuel cells.
The new nanocrystals have a "tetrahexahedral" structure, that is, a three-dimensional geometrical form having 24 equal facets and can be as much as four times more efficient than the existing commercial platinum catalysts.
Platinum has recently become even more valuable (it is more pricey than gold) due to new industrial applications that interrupted the jewelers' exclusivity in using this metal. New industrial applications use platinum as a catalyst in the catalytic converter, an optional (though often mandatory by law) component of the gasoline-fueled automobile exhaust system, or as a catalyst in hydrogen fuel cells and even in sophisticated sensors.
Though fuel cell could one day replace the internal combustion engine, there are still some technological problems to be overcome, like hydrogen economy and the efficiency of the chemical processes.
"If we are going to have a hydrogen economy, we will need better catalysts," said Zhong Lin Wang, a Regents Professor in the School of Materials Science and Engineering at the Georgia Institute of Technology. "This new shape for platinum catalyst nanoparticles greatly improves their activity. This work also demonstrates a new method for producing metallic nanocrystals with high-energy surfaces."
The new platinum nanocrystals are electrochemically produced using platinum nanospheres on a carbon substrate and can remain stable even at high temperatures.
Researchers were able to control their sizes by varying the number of cycles of "square wave" electrical potential applied to them and are able to control the size such that only 4.5 percent of the nanocrystals produced are larger or smaller than the target size.
"This electrochemical technique is vital to producing such tetrahexahedral platinum nanocrystals," said Shi-Gang Sun, an Eminent Professor in the College of Chemistry and Chemical Engineering at the Xiamen University in China. "The technique used to produce the new platinum nanostructures may also have applications to other catalytic metals."
The good thing is that the new nanocrystals can be as much as four times more catalytically active per unit area than existing commercial catalysts, but their present size, 20 times bigger than of those presently used still requires some refining of the processing techniques, which the researchers hope to be able to achieve in the coming years.
 14 DAY TRIAL //
14 DAY TRIAL //