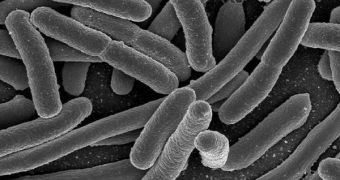
Most often, when bacteria create nasty infections inside human hosts, they defend themselves against antibiotics and the immune system via a coat of slimy proteins, known as a biofilm. These biofilms are notoriously hard to destroy, and, if they occur on implanted prosthetic devices, they are most often destroyed by removing the implant altogether. Now, scientists at the University of Michigan have developed a new tool to help them understand the mechanical behavior of biofilms, using nothing more than a specially designed microfluidic device, a class also known as labs-on-a-chip.
Essentially, what the new device does is measure the level of pressure the bacterial biofilms can withstand before collapsing and exposing the colonies underneath to the effects of antibiotics and immune system cells. UM Department of Emergency Medicine Associate Chair of Research John Younger explains that bacteria seldom live alone, and that it's precisely these large colonies that give biofilms their legendary strength. Details of the new microfluidic device and the way it counteracts these biofilms appear in a cover article, to be published in the July 7th edition of the journal Langmuir.
“If you want to understand biofilms and their life cycle, you need to consider their genetics, but also their mechanical properties. You need to think of biofilms as materials that respond to forces, because how they live in the environment depends on that response,” UM Associate Professor of Chemical Engineering, Macromolecular Science and Engineering Mike Solomon, who is also the senior author of the new research paper, says.
“We think a lot of host defense boils down to doing some kind of physical work on these materials, from commonplace events like hand-washing and coughing to more mysterious processes like removing them out of the bloodstream during a serious infection. You can study gene expression patterns as much as you want, but until you know when the materials will bend or break, you don't really know what the immune system has to do from a physical perspective to fight this opponent,” he continues.
The new channel-etched chip is made entirely up of a special type of polymer, and its structure is essential in granting it the ability to assess the resistance of biofilms. In their experiments, the team captured inside such a device minute amounts of bacteria from established colonies, numbering between 50 and 500 bacterial cells. These partial colonies created biofilms that were ten to 50 microns wide. For comparison, the human hair is, on average, 100 microns wide. The most amazing discovery that the team made was the fact that bioflms exhibited a “strain hardening response,” which means that the more you press on them, the harder they get.
 14 DAY TRIAL //
14 DAY TRIAL //