Since the beginnings of chemistry, reactions between some compounds have been found to need a little something called a catalyst in order to function. Catalysts are chemicals or other factors, such as pressure and heat, which must also be present between two or more chemicals, in order for reactions to take place. One good example is iron ore, which can only be manufactured into stainless steel under temperature and with the carbon and chromium chemicals.
Now, researchers at the Eindhoven University of Technology (TU/e) have devised a novel way of initiating chemical reactions, namely via mechanical forces. They have used such forces for the first time, and have managed to successfully control a catalytic reaction. The team have even succeeded in obtaining polymerization via this method. Polymerization refers to the construction of a polymer chain from monomers, which are small molecular building blocks suited for this process.
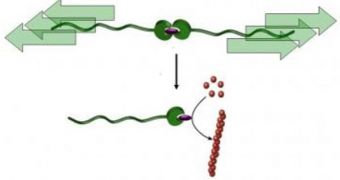
Dr. Alessio Piermattei, Dr. Karthik Sivasubramanian and Dr. Rint Sijbesma, from the TU/e Institute for Complex Molecular Systems (ICMS) and the Department of Chemical Engineering and Chemistry, have been the main researchers of the new technique, which they say will pave the way for the creation of smart materials. This new class of materials would be able to repair itself if mechanical pressure is applied on them. In a sketch they have released to the public (see image), the experts have proven that a dormant catalyst can be activated by pulling a molecular chain, called a “ripcord.” Once activated, the chemical reaction continues as usual.
A potential application for this find would be the creation of fabrics or metal alloys that would harden under stress. Imagine a sheet of metal that is tore by a saw or an ax. Once the initial shock rips through the metal, the sheet splits in two, but then the ripcord is activated and a chemical reaction ensues that welds the material back together, making it even tougher. There are, basically, no limits to the scientific areas in which these findings could be used. Frying pans, car bodies, fighter planes and space stations, all could benefit from such a protective shell.
 14 DAY TRIAL //
14 DAY TRIAL //