A group of scientists announces the development of a new material, to be used in filters that separate closely-related components of natural gas. If applied, this technique has the potential to drastically reduce the massive energy resources currently required for this task.
The team, which includes experts from the US National Institute of Standards and Technology (NIST), says that separating the components of natural gas is now an energy-intensive task, that leads to the creation of large amounts of useless byproducts.
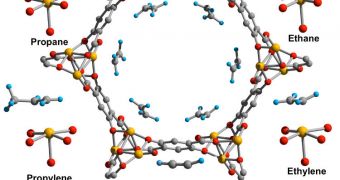
In a paper published in the March 30 issue of the top journal Science, the team explains that their material belongs to a class called metal-organic framework (MOF). These materials exhibit a very high surface area, and have a host of tunable properties.
These enable them to be used for a wide variety of applications, ranging from catalysis and gas storage to drug delivery. For this research, the group created an iron-based MOF, now called Fe-MOF-74.
It was developed in the lab of University of California in Berkeley (UCB) chemistry professor Jeffrey Long, and then underwent further analyses in labs at NIST and the Bragg Institute, a research center operated by the Australian Nuclear Science and Technology Organization (ANSTO).
According to scientists, natural gas contains a wide array of hydrocarbon molecules, of which only some are necessary for the production of fuel and plastic. But separating compounds such as propane and ethylene from each other, for example, is not a simple process.
The weight of these chemicals is very similar, so what researchers do now is cool both of them to temperatures below -100 degrees Celsius, and then wait for the heavier one to separate from the lighter ones. This takes up a lot of time and resources.
“A good percentage of the energy needed for separation goes to the cooling process. A material that can selectively adsorb light hydrocarbons could offer significant energy savings, making separation more economical,” NIST Center for Neutron Research postdoctoral fellow Wendy Queen explains.
The new material “works well at 45 degrees Celsius, which is closer to the temperature of hydrocarbons at some points in the distillation process,” the investigator goes on to explain.
“The upshot is that if we can bring the MOF to market as a filtration device, the energy-intensive cooling step potentially can be eliminated. We are now trying out metals other than iron in the MOF in case we can find one that works even better,” she adds.
 14 DAY TRIAL //
14 DAY TRIAL //