Officials with the US Food and Drug Administration (FDA) have recently approved the use of a new fluorescent dye in Alzheimer's detection. The substance binds to amyloid plaques in the brain, structures that are hallmarks of this neurodegenerative form of dementia.
The plaques are formed by accumulations of the beta-amyloid protein in the human brain, and they interfere with the normal functioning of neurons. Detecting elevated concentrations of this molecule before it starts forming plaques has been a focus of Alzheimer's research for many years.
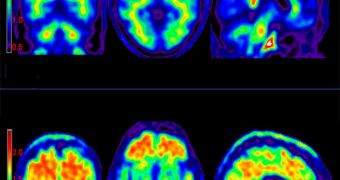
Now, investigators at the University of California in Los Angeles (UCLA) are able to use the new dye to reveal the plaques wherever they form. This means that doctors will soon have access to a test that they can apply to patients suspected of developing dementia.
Until now, the only way to be sure that a patient's brain was overrun by amyloid plaques was to perform an autopsy after the individual's death. With the new technique, it is possible to conduct such an assessment while they are still alive.
After the weakly radioactive dye is injected into the brain, a simple positron emission tomography (PET) scan can reveal their number and size, as well as their exact locations. When it hits the market, the dye will most likely be first used on people exhibiting cognitive decline or impairment.
Some of the most common signs of neurological problems include confusion and memory loss. As soon as clinicians detect these symptoms, they can recommend the new tests to the patients in question.
“A positive scan would add more weight to our bedside diagnosis. If a patient's cognitive impairment is purely due to depression, then a negative amyloid scan would help ascertain that,” UCLA Alzheimer's specialist, Liana Apostolova, explains.
Depression or side effects from various drugs could also trigger symptoms similar to cognitive decline, so discerning between their root causes is now a very complex procedure, which often fails to provide accurate results.
At this point, the new tests can only be used to rule out the condition. If a scan is positive, clinicians cannot really inform their patients that they have Alzheimer's, since the data on how to interpret these readings is not yet out there, Technology Review reports.
“I think [this] puts the clinician in a bind. The FDA nor the company says anything about the meaning of a positive scan. The data is not out there,” comments the Director of the Mayo Clinic Alzheimer's Disease Research Center, Ronald Petersen.
 14 DAY TRIAL //
14 DAY TRIAL //