At this time, many drugs against obesity and diabetes are available on the market, but most of them have their actions disrupted by an enzyme called PTP1B. Researchers in the United States now say that they can use this molecule as a target for a new type of treatment.
The team – which is based at the Cold Spring Harbor Laboratory (CSHL) – explains that this enzyme has been an obstacle to diabetes and obesity drugs for a long time, providing one of the most important technical hurdles to bringing effective chemicals to bear against these conditions
CSHL professor Nicholas Tonks FRS was the leader of the research group, and also the discoverer of PTP1B, back in 1988. At the time, he realized that the advanced protein played a critically-important role in the way cells' response to insulin was regulated by dedicated signaling pathways.
The hormone insulin is very important throughout the body, as it is responsible for ordering cells in the liver and muscle (and to a lesser extent in other organs) to harvest all available glucose from the bloodstream. The chemical is then stored in cells for later use.
Through this mechanism, insulin essentially regulates the metabolism of carbohydrates and fats. In diabetes, and some types of obesity, the hormone's actions are hampered, which leads to numerous side-effects on health.
“Type 2 Diabetes, the most common form, is a disease of insulin resistance. In the disease state, an insulin molecule docks at a cell, but the cellular mechanism that sends its signal does not work properly,” Tonks explains
“This is why simply adding more insulin will not solve the problem. What you really need to do is to promote insulin signaling within the cell – to favor the phosphorylation events triggered by the insulin receptor,” he adds.
Mouse models in which the gene encoding for PTP1B is knocked out are extremely resistant to obesity, even if fed diets high on fat and carbohydrates. They are also very resistant to developing diabetes, and are more susceptible to being influenced positively by insulin.
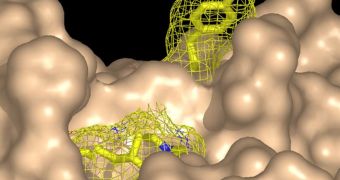
A drug-like inhibitor of PTP1B could therefore have a similar effect in humans as well, the CSHL team explains. “The development of molecules that block the active site [on the enzyme], and thereby inhibit the function of an enzyme, is a standard strategy for drug design,” Tonks adds.
In a paper published in the September 29 issue of the journal Cell, the team presents a new target on the surface of the enzyme that can be used to inhibit its function. This approach side-steps some of the most important challenges associated with using other, previously-known active regions.
 14 DAY TRIAL //
14 DAY TRIAL //