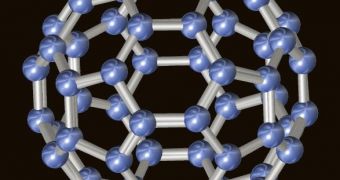
The fullerenes, discovered in 1985 by researchers at Rice University, are a family of carbon allotropes named after Richard Buckminster Fuller and are sometimes called buckyballs. They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Cylindrical fullerenes are called Carbon nanotubes or buckytubes. Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
The original buckyball, is a cage-shaped molecule of 60 carbon atoms. The boron buckyball, developed by Rice University scientists, consists entirely of boron atoms and is structurally similar to the original C60 fullerene, but it has an additional atom in the centre of each hexagon, which significantly increases stability.
Boris Yakobson, professor of mechanical engineering and materials science and of chemistry, who conducted the research along with his associates Nevill Gonzalez Szwacki and Arta Sadrzadeh, said: "This is the first prediction of its possible existence".
"This has not been observed or even conceived of before. We do hope it may lead to a significant breakthrough," Prof. Yakobson said of the boron buckyball, or B80.
In the earliest stages of their work, the team attempted to build a "buckyball" using silicon atoms but determined that it would collapse on itself. Their search for another possible atom led them on a short trip across the periodic table.
As initial work with 60 boron atoms failed to create a hollow ball that would hold its form, another boron atom was placed into the center of each hexagon for added stability.
"Boron is nearby (one atomic unit from carbon). One reason we tried it was because of proximity. Boron also has the ability to catenate, to stick together better, than other atoms, which also made it appealing," said Prof. Yakobson.
"Bob (Curl) said with a chuckle that it was more of a 'buckyball' than his buckyball, the reason being that C60 was named for famed architect Buckminster Fuller, because the buckyball looked like conjoined geodesic domes, a structure that Fuller had invented. When Fuller made his domes, he made them from triangles because hexagons would collapse. In B80, we fill the hexagon with one more atom, making triangles."
"However, it is too early to speculate whether the boron buckyball will prove to be equally or more useful than its Nobel Prize-winning sibling. All we know is that it's a very logical, very stable structure likely to exist," Prof Yakobson said.
"But this opens up a whole new direction, a whole new continent to explore. There should be a strong effort to find it experimentally. That may not be an easy path, but we gave them a good road map," he added.
As for the practical applications, there are possible uses of fullerenes in armor or for potential medicinal use: binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma.
 14 DAY TRIAL //
14 DAY TRIAL //