A team of researchers in the United Kingdom is currently working towards testing a two-step approach to treating glioblastoma, the most common and dangerous form of brain cancer. The new treatment option would make use of both fluorescent markers and specialized drug wafers.
Glioblastoma is a type of malignant brain tumor that kills most patients in less than 15 months from the time of diagnostic. Doctors need to act swiftly if they want to save people's lives, but the cancer is extremely aggressive and difficult to pin down.
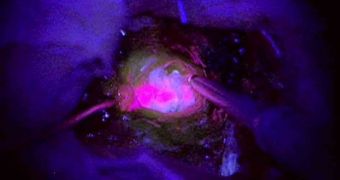
This is why even the most successful surgeries oftentimes do not manage to remove all traces of the cancer. This is why researchers are currently thinking about using glow-in-the-dark markers, which would expose the exact tissues that are affected by glioblastoma.
The Samantha Dickson Brain Tumor Trust and Cancer Research UK are jointly funding the new, multi-center phase II clinical trial, which is being coordinated by University of Cambridge HEFCE clinical senior lecturer Dr. Colin Watts.
About 60 patients have already enrolled in the new clinical trials, whose role is to assess how well the two proposed treatments work together. The fluorescent chemical – called 5-ALA (5-amino-levulinic acid) – is important because it glows under ultraviolet light.
During surgery, doctors can get a better view of the tumor's edge. This in turn enables them to extirpate the entire structure with a higher degree of accuracy, boosting patients' chances of survival years later.
In the second step of the newly-proposed treatment, patients would administer carmustine-laden wafers straight at the locations where the bulk of the tumors was taken out. Essentially, doctors replace the affected tissues with chemotherapy drugs.
Small, individual tumor cells that doctors cannot extract should be killed by the drug. In theory, at least, this should work remarkably well. Some of the centers where tests will be carried out include King’s College Hospital and the National Hospital for Neurology & Neurosurgery, both in the UK.
“I strongly feel that our best opportunity to progress further is to emphasize funding of lab-based research and innovative trials and the GALA-5 trial is a significant step forward in making this a reality,” Watts explains.
“I am delighted to see this partnership between Samantha Dickson Brain Tumour Trust and Cancer Research UK, which really make a difference and allow more trials and clinicians to be supported,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //