Lithium-ion batteries have over the past few years became the best choice for most consumer applications such as laptop and mobile phone batteries. The rate at which they are constantly improved has started to decline, mostly because the existing manufacturing technology has reached its limits. But experts at the Stanford University are about to get the technology going again, as they recently presented the idea of using nanowires inside future generations of batteries, so as to improve their performance, longevity, and reliability, Technology Review reports.
SU materials science and engineering professor Yi Cui announced the development of a new nanowire electrode, which can be used to store up to six times as much charge as an average lithium-ion battery with graphite electrodes. This could have vast implications for the auto industry, as new generations of electric car battery packs could propel the vehicles for many more miles than existing batteries can today. Cui explains that the new material exploits the properties of both silicon – which is the best possible material for storing charge in battery packs, but has unsustainable usage requirements – and graphite, all in the form of nanowires.
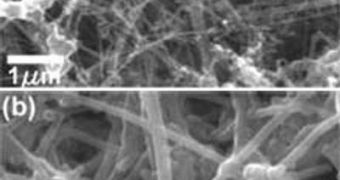
The new electrodes are in fact made from amorphous silicon, which is deposited on carbon nanowires. Silicon is known to be able to store about ten times more ions than graphite can, but it's notorious for swelling up when it does so, up to four times its original size. Additionally, it cracks up after only a few recharging cycles. While graphite anodes store less than 360 milliamp hours per gram, the new material can hold a whooping 2,000 milliamp hours per gram, the SU team says. “Lithium ions can also get absorbed into carbon, but the volume expansion of carbon is 10 percent or smaller, so it provides a stable backbone,” Cui explains.
“Carbon nanofiber is already commercially available and you can produce tons. The coating process could be made a lot faster and is easy for large-scale manufacturing,” the expert states. Additionally, the production process is not as demanding as the one needed to produce pure silicon nanowires, and also requires smaller temperatures. The team mentioned that they tried to use pure silicon wires, and that they had the ability to triple the storage capacity of graphite electrodes, but said that the wires were only stable for 20 charge cycles.
 14 DAY TRIAL //
14 DAY TRIAL //