Chemistry professor Tim Long's research group, students affiliated with the Macromolecule and Interfaces Institute (MII) at Virginia Tech, and the U.S. Army Research Laboratory, presented a research at the national meeting of the American Chemical Society in Chicago, at the interface between nanotechnology and biotechnology.
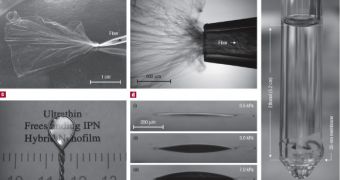
Thin film composite membranes are semipermeable membranes manufactured for use in water purification, desalination systems, batteries, fuel cells, but also for medical purposes.
For example, silicon nanomembranes are thin silicon layers that can be released from their substrates and redeposited on other substrates. Such membranes are flexible and can be coherently strained, producing tensile strained silicon without the need for dislocation-driven relaxation of a SiGe virtual substrate.
The researchers announced last year that they had created a nanostructured membrane that incorporates DNA base pairs in order to impart molecular recognition and binding ability to the synthetic material. This year they will show for the first time that these new films, membranes, and elastomers are compatible with diverse organic and inorganic molecules and will adopt properties of the guest molecules.
They effectively succeeded in creating a block copolymer, where different monomers are linked in a sequential manner and form a nanostructured film. They used adenine and thymine nucleotides, two of the four DNA base pairs that recognize each other.
Then the researchers experimented with different kinds of guest molecules with complementary hydrogen bonding sites (hydrogen has a low energy attraction to many types of atoms)
Due to this low energy attraction, means the guest molecules are widely dispersed throughout the membrane, which then takes on the properties of the guest molecules. "For example," said Long, "if the guest molecules have ionic sites (sites with positive and negative charges), you will be able to transfer water through a film because you would have ion channels at the nanoscale. It's similar to the way a cell membrane works to control the flow of specific ions into a cell. You can create protective clothing -- against chemicals -- that would still allow water vapor through."
One application could be introducing salts such as ordinary table salt (NaCl), into a block copolymer template, that would permit the placement of the salts at the nanometer dimension to create channels of salts at that are not visible with the human eye, but act as a roadway for the transport of water molecules.
 14 DAY TRIAL //
14 DAY TRIAL //