University of California in Berkeley (UCB) investigators say that a new computer model they've developed has recently been able to identify a series of materials that could make carbon capture and storage (CCS) technologies cheaper and more feasible for implementation at a large scale.
These technologies are essential for reducing the amount of greenhouse gases that humans are currently releasing into the atmosphere. Carbon dioxide is undoubtedly the most important, even though it is not the most dangerous one. Billions of tons of CO2 are released into the air annually.
Vehicles, airplanes and fossil fuel-based power plants are the most important sources of CO2. By installing special equipment on power plant smokestacks, it may be possible to trap carbon in exhaust fumes before it is released into the atmosphere.
Doing so is currently an energy- and resource-intensive process, one that researchers hypothesized could be made less demanding through the development of new materials. The UCB team's model proposes materials that meet these criteria.
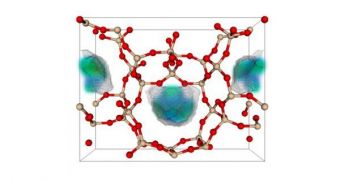
Experts here say that zeolites and metal oxide frameworks (MOF) could significantly reduce the costs of implementing CCS technologies in existing facilities. They add that the mount of parasitic energy consumed by existing systems – about one third of what power stations produce – could decrease, too.
The purpose of zeolites and MOF is to capture as much carbon as possible. After this stage in the process, the greenhouse gas would be deposited underground, in specially prepared chambers.
“The current on-the-shelf process of carbon capture has problems, including environmental ones, if you do it on a large scale,” explains Chancellor's professor Berend Smit, who is based at both the Departments of Chemical and Biomolecular Engineering, and the Department of Chemistry at UCB.
“Our calculations show that we can reduce the parasitic energy costs of carbon capture by 30 percent with these types of materials, which should encourage the industry and academics to look at them,” adds the scientist, quoted by e! Science News.
Smit also holds an appointment as a faculty senior scientist in the Materials Sciences Division at the US Department of Energy's (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab).
Details of the new study appear in the May 27 advanced online issue of the top scientific journal Nature Materials.
 14 DAY TRIAL //
14 DAY TRIAL //