Scientists at the European Molecular Biology Laboratory (EMBL), in Monterotondo, Italy, were recently somewhat disappointed at the result of one of their investigations, before they realized that they had in fact stumbled upon something great. The team essentially found that white blood cells known as macrophages played a huge role in healing injured muscles, and also that the entire process was controlled by a molecular switch that was also identified. From now on, scientists could use these results to try new treatments for a number of medical conditions, PhysOrg reports.
According to the paper detailing the finds, which appears in the latest issue of the respected journal Proceedings of the National Academy of Sciences, sport-related injuries, and even diseases such as the Duchenne muscular dystrophy, could all be treated faster than currently possible in the future, with the help of the new finds. The discovery was made even more amazing by the fact that common knowledge dictated macrophages were usually involved in eliminating pathogens by engulfing them.
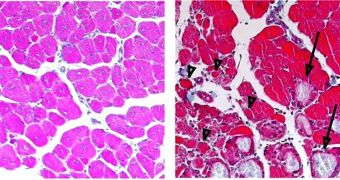
Whenever an infection occurs, macrophages are drawn to “ground zero,” where they start seeking out the intruders, bind to them, and eat them. Afterwards, they play a key role in eliminating dead cells, and also in releasing pro-inflammatory factors, which are able to fend off infection. After the infected area has been cleared and checked, macrophages release anti-inflammatory factors, which start repairing the damage caused by the confrontation. The EMBL team, led by Claus Nerlov and Nadia Rosenthal, proved that this shifting process, known as “macrophage polarization,” was essential to muscle regeneration after an injury.
“There seems to be this point of no return: if macrophages don’t make this switch, then the muscle won’t repair itself – you just end up with scar, instead of new tissue,” Rosenthal says. The find came as a surprise, because the initial research was not focused on muscle regeneration, but on the development of immune-system cells, while the production of a protein known as C/EBPb was impaired. The protein was known to be produced in increasingly large quantities as inflammation progressed. In the rat studies, Nerlov's team obtained no conclusive results, but noticed that muscle injuries no longer healed properly, but rather became scarred. Rosenthal's group was then alerted of the find.
“From a medical point of view, it would seem that the trick to improve muscle repair is finding a way to increase C/EBPb production and keep it high. If we can now figure out exactly which key genes C/EBPb controls, that will give us even more potential targets,” Rosenthal concludes.
 14 DAY TRIAL //
14 DAY TRIAL //