Scientists in the United States announce the development of a new bioengineering technique, which allows them to create strings of living cells and nanoscale filaments. These structures can then be used to bridge gaps at a macroscopic scale, which means that they could possibly underlie a new generation of advanced therapies against heart diseases and spinal cord injuries. Additionally, the group believes, the constructs could also help create better and more efficient artificial muscles. The research team behind the achievement is based at the Northwestern University in Illinois, Chemistry World reports.
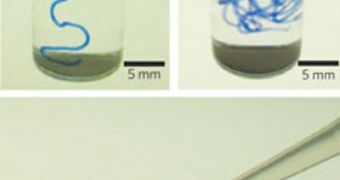
“We have discovered a way to align huge arrays of nano-sized filaments and living cells over macroscopic distances. The arrays form a noodle-shaped gel that could potentially be implanted where cell alignment is important, such as in cardiac or nervous tissue,” explains Northwestern expert Sam Stupp, the leader of the new investigation. The method the group developed is fairly ingenious in its approach. A solution of amphiphilic peptides is used as a starting point. These molecules are known for their ability to self-assemble, which makes them ideal for this type of studies.
The peptides turn into nanofiber bundles after they are heated up, generating liquid crystals. After this happens, a pipette is used to squeeze the solution in a mix of water and sodium chloride (NaCl) or calcium chloride (CaCl2). “When the liquid crystal is drawn by hand over the salty medium, all the domains align in the same direction and calcium ions freeze the alignment in noodle-like gel,” Stupp says. By varying the diameter of the pipette, the researchers can easily control the thickness of the living cell strings. The structures are not toxic for the body, but are in turn flexible. Researchers say they can easily stretch, bend and roll.
“In terms of regenerative medicine, we still need to find ways to better control how stem cells develop into tissue. And for applications such as heart surgery, we need to find ways to produce thick muscle tissue and integrate it smoothly with the rest of the heart,” says Duke University cardiac tissue engineering expert Nenad Bursac. “This is an innovative and exciting approach,” he adds. “This work represents an important step forward in creating artificial muscles. It also represents a new tissue engineering scaffold that may help to repair muscle or nerve damage in patients,” concludes University of British Columbia expert Hongbin Li, who was in charge of a Canadian team that managed to create artificial proteins that mimic muscle tissue.
 14 DAY TRIAL //
14 DAY TRIAL //