Although they are highly reactive with other chemical elements and substances, lithium and beryllium do not bind together under normal atmospheric conditions. A team of Cornell researchers predict however that, while subjected to high levels of pressure, two of universe's lightest elements could, in fact, create stable alloys that would be suitable for superconductivity applications.
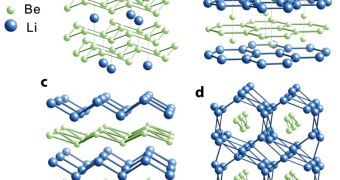
Very little is known about the properties of materials created under extreme levels of pressure, so the National Science Foundation initiated a program called Inorganic, Bioinorganic and Organometallic Chemistry in order to simulate such interactions. Calculations reveal that lithium and beryllium would react with each other on a 1:1 ratio, in order to create a stable alloy, LiBe compound. Three dimensional models predict that a two dimensional electron gas layer would compress the compound into a three dimension crystal and remain so for an indefinite period of time.
Co-author of the study, Roald Hoffmann, Noble Prize for chemistry laureat in 1981, and Cornell Frank H.T. Rhodes, Professor in Human Letters Emeritus, compare the compound with a layer cake subjected to relatively high pressure. During this time the layers would mix together to form a random uniform layer of cream.
On the other hand, Neil Ashcroft and Cornell Horace White, Professor of Physics Emeritus, argue that lithium and beryllium layers under pressures between 5 to 10 times that at which diamonds form, would push the lithium electron gas layer into the close proximity of the beryllium layer, in order to create two dimensional gas layers. The two dimensional behavior between two simple chemical elements is absolutely remarkable and is mostly related to their simple configuration, said Ashcroft.
It is predicted that LiBe compound would have good superconductive properties, but it is only a speculation for the time being, because theoretical models do not seem to confirm this presumption. The phenomenon, however, could be tested in real life as physicists say that manufacturing the LiBe alloy is relatively easy. Superconductivity is manifested by materials while subjected to very low temperatures during which time they tend to experience a close to zero electrical resistance.
 14 DAY TRIAL //
14 DAY TRIAL //