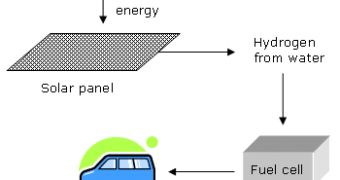
The alternatives to fossil fuels such as wind power or solar power have a down-side: they are not mobile. In order to fix this problem, one can use the clean technology to charge batteries that one could then carry around. Among the most promising such clean batteries are the hydrogen fuel cells.
Essentially, such a fuel cell combines oxygen with hydrogen producing water and electrical energy. One could use the solar energy to create the opposite process - consuming the energy for obtaining hydrogen from water. The hydrogen is then used in fuel cells. Thus, by using this combination, one virtually stores the solar energy as hydrogen in the fuel cell.
In order to make this entire process as efficient as possible, one has on one hand to transform as much solar energy into electric energy (which is used to hydrolyze the water and obtain the hydrogen) and, on the other hand, obtain as much energy from the fuel cell. Moreover, the fuel cells have to be as reliable as possible.
The reaction between oxygen and hydrogen that takes place inside a fuel cell is catalyzed. A catalyst is a chemical substance that speeds up a reaction. Thus, the main issue of constructing an efficient fuel cell is to find the best catalyst. The more you can speed up the reaction the more power you get.
Insofar, the most common catalysts use platinum - which is of course a very expensive precious metal. Platinum is used because most other substances don't resist the highly acidic environment inside the fuel cell (the high acidity exists because of the hydrogen) - one wants the hydrogen to react with the oxygen and not with the materials of the fuel cell or with the catalyst itself!
Scientists at Los Alamos National Laboratory have now developed a new class of hydrogen fuel-cell catalysts that exhibit very promising activity and stability. Although the new catalyst isn't as efficient as the platinum-based catalysts it is more efficient than other non-platinum catalysts and it also has a remarkable durability.
Rajesh Bashyam and Piotr Zelenay have subjected the composite material (made of cobalt, polymer and carbon) to over one hundred hours of continuous testing. Such a result was never obtained before with non-precious metal catalysts.
"Besides being made of inexpensive and environmentally benign materials," said Zelenay, "the chief advantage of these composite catalysts for oxygen reduction is that they can operate in the acidic environment of the polymer electrolyte fuel cell."
According to Ken Stroh, program manager for the Los Alamos fuel-cell effort, "the two biggest obstacles in making a commercially viable fuel cell have traditionally been high cost and inadequate durability. Our focus at Los Alamos is to attack those obstacles as a system in which you simultaneously strive for lower costs and higher durability."
Bashyam and Zelenay are now trying a variety of composites attempting to develop new catalysts and electrode structures that would increase the current output from fuel cells.
 14 DAY TRIAL //
14 DAY TRIAL //