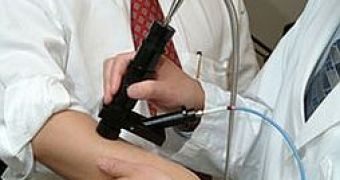
British Columbia Cancer Agency (BCCA) scientists developed a new handheld device, licensed to Verisante Technology, that provides instant data about the molecular structure of moles, telling dermatologists whether a biopsy is necessary or not.
Melanoma is the most deadly form of skin cancer, and the only ones that can detect it are dermatologists, who decide which moles to biopsy.
This device, called the Verisante Aura, makes the detection process much more reliable, since it uses Raman spectroscopy, a technique that identifies molecules by their vibrational state.
Held above a mole, the instrument scans for relative concentrations typical of melanoma, and gives back a verdict within seconds.
The work on this project started eleven years ago, when BCCA dermatologists were studying whether certain skin diseases could be detected by their unique spectral characteristics.
Raman spectroscopy is based upon laser lights that change the vibrational state of the bonds within molecules, causing a shift in the light, that is reflected back to the sensor.
It is the magnitude and direction of this shift that reveal the kind of molecules contained in the sample, as well as their concentration.
Co-inventor David McLean, a professor of medicine at the University of British Columbia, says that the team “thought that because the Raman spectrum shift was a direct reflection of the molecules targeted, and because different skin lesions would have different molecules in differing concentrations, it should produce a diagnostic signature.”
So even if a mole looks benign, the comparison of its spectral signature to examples of melanoma and other skin diseases from a database, will provide a certain diagnosis.
This should help dermatologists decide whether to biopsy or not, and it could also be used by non-dermatologists, in areas like rural Canada.
Verisante conducted a successful small clinical trial and is now assessing the results of another larger trial, involving 1,000 moles.
Later this year, the company plans to obtain approval from the US Food and Drug Administration and Canada Health, Technology Review reports.
However, there could be some concerns with this device, such as the matter of false negatives – a mole considered harmless turns out to be a melanoma, or dermatologists misusing the device for diagnosing melanoma instead of deciding the necessity of a biopsy.
Despite these regulatory dilemmas, and whatever US and Canadian regulatory agencies decide, Darrell Rigel, a professor of dermatology at NYU's Langone Medical Center, and former president of the American Academy of Dermatology, says there is a need for devices that can help identify melanoma.
“It's a challenge to diagnose melanoma clinically. It's subjective,” he says.
“And while it's not so difficult with one spot, many people have lots of spots—how do you decide what to biopsy?”
Patients whose melanoma isn't detected early, have a life expectancy under one year, and the rates of this disease are sky-high: in 1935, one in 1,500 Americans was estimated to get melanoma in their lifetime, whereas today, the estimates turn around one in 58 people.
Watch a video demonstration of the device at CTV.
 14 DAY TRIAL //
14 DAY TRIAL //