A team of scientists from the Florida campus of The Scripps Research Institute have discovered a molecular interaction between a structural hepatitis C virus protein (HCV) and a protein ensuring its viral replication.
This could lead to a new method of stopping the production of the virus and to an improved therapeutic target for the development of drugs against hepatitis C.
The new research stresses the importance of the viral protein called the 'core', which leads the infectious HCV particle assembly.
The hepatitis C virus is the cause of an epidemic of liver cirrhosis and cancer, infecting between 130 and 170 million people worldwide.
Current HCV treatments only work in some cases, so researchers are always open to the possibility of finding alternative molecular mechanisms that could turn into possible drug targets.
The main issue is that the hepatitis C virus has an amazing ability to mutate, reaching rates as high as one million times those of DNA viruses like the herpes virus.
So, Scripps Florida Professor Donny Strosberg, leader of the study, has been focusing his research on the core protein – the most conserved protein among all HCV genotypes.
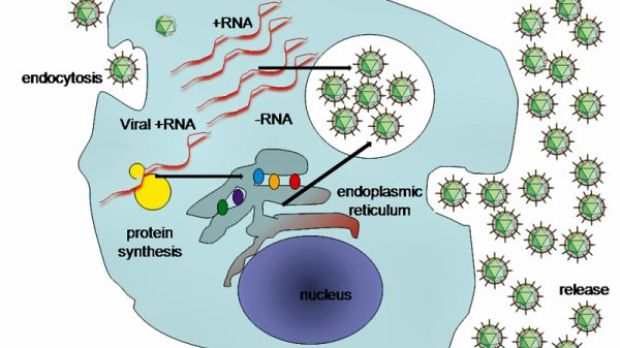
Core is very important for the fabrication of the hepatitis C nucleocapsid or capsid; this virus genome is protected by a protein coat, and it is very important for the formation of infectious viral particles.
During the assembly and the release of the infectious virus and in the disassembly of viral particles when entering host cells, core plays a crucial part in the HCV cycle, by interacting with several structural and non-structural viral proteins.
The researchers believe that core also interacts with several cellular proteins, which can possibly contribute to inhibiting several host defense mechanisms, thus activating oncogenic pathways.
Strosberg said that “while our finding that the HCV core interacts with the non-structural helicase protein was not totally unexpected, this had not really been confirmed until this study.
“But the most exciting part is that small molecule inhibitors of dimerization [the joining of two identical subunits] of core actually inhibit interaction between core and helicase, thus possibly preventing production of an infectious viral particle.”
Last year, he developed a new quantitative test for monitoring these interactions between proteins, in an attempt to find the inhibitors of the core dimerization, and possibly block virus production.
Strosberg and his colleagues uncovered peptides derived from the core protein of hepatitis C that besides inhibiting dimerization, also tackled the production of the virus itself.
Based on the findings of that study, the new research used SN201 – one of the non-peptidic small organic molecules that inhibit HCV production.
This time, Strosberg and his colleagues focused on non-structural proteins that provide functions relating to HCV production, especially NS3 helicase.
Their research strongly suggests that this protein participates in the assembly and production of infectious viral particles.
The new study was supported by the state of Florida, The Factor Foundation, and the National Institutes of Health, and it was published in the Journal of General Virology.
 14 DAY TRIAL //
14 DAY TRIAL //