The final form of predicted ice has been successfully obtained in the laboratory. After years of research, scientists at the University of Oxford, in England, managed to create ice XV, a never-before-reached form of ice, with a special form of molecular layout. Thus far, 16 new types of the stuff have been discovered, including two forms of ice I. The most common type is Ih, with the “h” standing for hexagonal. This accounts for the six-sided symmetry of all snowflakes, Wired reports.
“We have removed the question mark from the phase diagram of water,” Oxford expert Christoph Salzmann says. He is the co-author of a new paper detailing the find, published online in the September 2nd issue of the respected scientific journal Physical Review Letters. The scientist adds that the types of ice are determined by how close the molecules of water pack themselves together, and also by the overall layout of this arrangement.
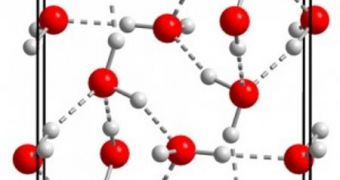
A phase diagram is a predicted, or already-established, table that dictates how molecules of a certain substance will behave at various temperatures and pressures. With the new experiments, the team finally erased the last question marks that anyone might have still kept on the water phase diagram. The new substance was obtained from ice VI, by lowering its temperature to about 130 degrees Kelvin, around -143º Celsius. The mix was then subjected to a gigapascal, or 10,000 atmospheres, of pressure.
Under these circumstances, the hydrogen bonds in ice VI snapped, and rearranged themselves in a tighter, highly organized configuration, in tune with predictions of ice XV. According to the Oxford team, the newly obtained material makes regular, rock-hard, ice shelf ice seem very fluffy. The good news is that the new variety may not be something that can only be obtained under precisely controlled lab conditions, Salzmann says.
The expert believes that it may also occur naturally, in the tightly packed cores of icy exoplanets and moons. The reason why ice XV does not form on the Earth is very straightforward. The only places on our planet that have the kind of pressure needed for hydrogen bonds to snap are also the hottest.
 14 DAY TRIAL //
14 DAY TRIAL //