Hydrogen fuel for vehicles would be the solution to cut off carbon dioxide pollution, with the environmental issues that it triggers, like global warming, because the only waste would be water, but current technology is extremely expensive and still developing.
A new research at U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) and Argonne National Laboratory (ANL) have developed a new platinum-nickel alloy proven to be by far the most active oxygen-reducing catalyst ever reported.
The slow speed of oxygen-reduction on the cathode (the positively charged electrode) has been a problem for the development of the polymer electrolyte membrane (PEM) for hydrogen fuel cells. "The existing limitations facing PEM fuel cell technology applications in the transportation sector could be eliminated with the development of stable cathode catalysts with several orders of magnitude increase in activity over today's state-of-the-art catalysts, and that is what our discovery has the potential to provide," said Vojislav Stamenkovic, from the Materials Sciences Division of both Berkeley Lab and Argonne.
The new platinum-nickel alloy rose the catalytic activity of a fuel cell cathode by an amazing 90 times compared to current platinum-carbon cathode catalysts. "This surface sets a new bar for catalytic activity in PEM fuel cells and makes it feasible to meet U.S. Department of Energy (DOE) targets for platinum-specific power densities without a loss in cell voltage," Stamenkovic said.
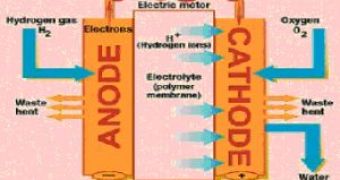
PEM ("proton exchange membrane") fuel cells fueled by hydrogen can deliver high power in small, light-weight device. PEM fuel cells are not batteries, they do not require recharging, just hydrogen fueling and atmospheric oxygen.
NASA employs them, but by now they remain too expensive for market availability, especially due to the platinum component, used as the cathode catalyst. A PEM fuel cell contains a positive electrode (cathode) which makes oxygen reduction and anode (the negative electrode) which causes the reduction. "Massive application of PEM fuel cells as the basis for a renewable hydrogen-based energy economy is a leading concept for meeting global energy needs," said Stamenkovic.
Pure platinum is a very good catalyst, but it's extremely expensive and easy degraded by by-products, like hydroxide ions.
That's why scientists have searched for a platinum alloy in combination with a surface enrichment technique: the cathode is covered with a layer of platinum, and beneath it, there's a platinum alloy with a non-precious metal, like the nickel or cobalt. The subsurface alloy must improve the qualities of the platinum layer.
The team made pure crystals of platinum-nickel alloys varying in a range of atomic lattice structures in an ultra-high vacuum (UHV) chamber, and measured crystals' qualities using surface-sensitive probes and electrochemical techniques.
The platinum-nickel alloy configuration Pt3Ni (111) was proven the most reactive for ORR activity among those that have ever been found on a cathode catalyst: 10 times better than a single crystal surface of pure platinum(111), and 90 times better than platinum-carbon. This configuration means that the subsurface layer is made up of similar numbers of platinum and nickel atoms.
The layers underneath the top two layers were made of three atoms of platinum at every atom of nickel. Because the alloy buffers against hydroxide and other platinum-binding molecules, it also impedes the degradation of the cathode surface. "We have identified a cathode surface that is capable of achieving and even exceeding the target for catalytic activity, with improved stability for the cathodic reaction in fuel cells," said Stamenkovic.
"Although the platinum-nickel alloy itself is well-known, we were able to control and tune key parameters which enabled us to make this discovery. Our study demonstrates the potential of new analytical tools for characterizing nanoscale surfaces in order to fine-tune their properties in a desired direction."
"The next step will be to engineer nanoparticle catalysts with electronic and morphological properties that mimic the surfaces of pure single crystals of Pt3Ni (111)."
 14 DAY TRIAL //
14 DAY TRIAL //