One of the biggest challenges opposing the wide-scale introduction of hydrogen cars on public roads is the fact that hydrogen fuel sources are still fairly large and cumbersome, and they cannot be easily carried around. For long voyages in fuel cell-powered vehicles, having a spare is a must, but the large dimensions that these replacements now have make this impossible.
Now, experts at the Stanford University have managed to discover that a hydrogen-packed material, which is, in fact, a form of ammonia borane (or borazane), may be able to allow researchers to move past the fuel cell-size and -handling problem.
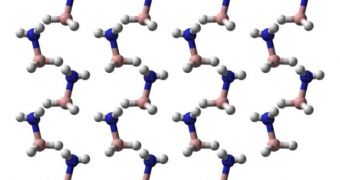
Stanford researcher Yu Lin is the lead author of a new scientific paper detailing the find, which has been published in this week's online issue of the journal Proceedings of the National Academy of Sciences (PNAS). “Including the hydrogen already stored in ammonia borane, this new material can store around 30 weight percent in total,” Lin says. A molecule of ammonia borane usually consists of six hydrogen atoms, which is the main reason why it came to attention as a source of fuel in the first place.
The new compound, dubbed ammonia borane-hydrogen (ABH), is able to store three times the amount of fuel requested by the United States Department of Energy. The DOE has asked all car manufacturers constructing fuel cell-powered vehicles to install back-up systems in their cars, which would allow them to store about nine percent of the hydrogen used, according to weight. ABH is able to store 27 percent of the hydrogen mass, which means that we may be hearing a lot more about this new material in the coming years, as soon as experts find a sustainable and economic way of synthesizing it in large quantities.
The problem with Lin's research is that the hydrogen he forcefully added to the ammonia borane was inserted into the existing compound at a pressure roughly equivalent to 60,000 times that found on the surface of the Earth. It goes without saying that, if the compound is to be used in cars, it has to perform the same reaction at normal temperatures and pressures, which may prove to be the most complex part of the research process.
 14 DAY TRIAL //
14 DAY TRIAL //