They say a mother is more than a father. That is true, as a mother invests more of its biology in the offspring anyway. During egg development (oogenesis), the mother deposits in it large amounts of messenger RNAs (mRNAs) and proteins. These mRNAs take over after the sperm did its job to orchestrate the emergence of a new life in the earliest stages of embryogenesis. After that phase, the embryo turns on its own genome, with both mother's and father's genes, renouncing to the pre-existing maternal mRNAs.
The maternal-to-zygotic transition (MZT) will install when the fertilized egg (zygote) has integrated the pre-existing maternal mRNA transcripts with signals that turn on its own genes. In that moment, the zygote has its own life; a new life has emerged.
But how this occurs has been a puzzle till the new research made on fruit fly, Drosophila melanogaster. The researchers detected classes of zygotically and maternally expressed genes, revealing how the embryo combines zygotic transcription with maternal mRNA degradation to turn on local genes expression necessary for the auto-organization of the early embryo.
The fertilized egg has just one membrane filled with nuclei that divide repeatedly till each one is wrapped in its own membrane forming separate cells. After the nuclear division 13, the zygotic genome is already in control.
Researchers had assigned increased transcripts as zygotic (if higher mRNA signals is seen as increased transcription), stable transcripts as from mother's mRNA, and decreasing transcripts as degraded maternal transcripts. But this type of approach cannot estimate concomitant degradation and transcription: mRNA can be similar even with a zygotically active genome if maternal transcripts decrease at the same rhythm.
But the research team developed a technique distinguishing between the two. First, the researchers assessed gene activation at 1-hour intervals for 3 hours after the initiation of the embryogenesis: the unfertilized egg (0-1 hour) and nuclear cycle 14 (2-3 hours), soon after the zygotic genome takes control (the midblastula transition). Microarray analysis of the gene activity at the onset of zygotic transcription showed that transcription and post-transcription were both active.
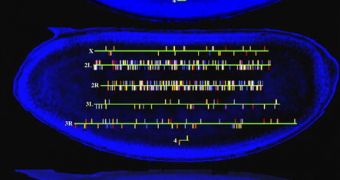
The researchers chose fruit fly as its embryos can survive enough when losing entire chromosomes and display distinct morphologies when chromosomes patches are removed, permitting the investigation of the zygotic activation. They compared embryos missing 20% of their genes with normal ones. As the maternal mRNA for those genes lacked, the correspondent mRNA for those genes in the embryo could come only from zygotic transcription. The team also analyzed mRNA profiles in embryos lacking entire chromosomes. The researchers detected easily mRNAs linked to the presence of a particular chromosome and their origin. The mRNA levels of these mutants were compared to that from untreated embryos.
For about a third of the genes, zygotic transcription compensated for the degraded maternal mRNA, and as a result, the levels at cycle 14 were kept constant, revealing that assessing the dropping of mRNA level was not a reliable method of assessing zygotic activity. Roughly 33 % of the genes in the zygote had no maternal counterparts, most being transcription factors. The newly transcribed mRNA could have roles the maternal genes do not possess. The maternal transcripts and zygotic gene transcription were proven to have distinct regulatory elements.
The turning on of the zygotic genome (which represents about 20% of the mRNA at cycle 14) starts when an egg protein, stored during oogenesis, activates the regulatory motif of the early zygotic genes.
Further researches will show if this pattern is valid also for other animal species.
 14 DAY TRIAL //
14 DAY TRIAL //