Scientists at the Yale University have recently developed a new method of observing how immune-system cells track invading bacteria, before finally catching up with them, and beginning a confrontation. In a paper published in the November 15 advanced online issue of the respected scientific journal Nature Methods, the team details how it managed to create artificial bacterial traces, which allowed them to study the behavior of neutrophils in response.
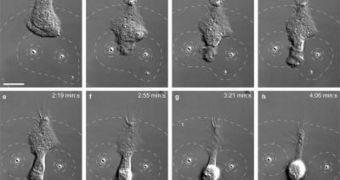
As bacteria enter the body, they begin to secrete various molecules, which are then left behind the moving pathogen, like a trail of bread crumbs. The immune system picks up on that trail, and sends various types of cells in pursuit. This mechanism was discovered some time ago, but researchers had no idea how it worked in detail. The new method allows for the creation of artificial bacterial trails, which can be modified in three dimensions over time. In the experiments, neutrophils – a type of white blood cells – are set on the tracks, and then their motions get analyzed.
“By fusing recent advances in optical and materials science, we've developed a new approach to control chemical microenvironments with light. Until now, people have used optical tweezers to move physical objects. We've demonstrated that they can also be used to manipulate chemical gradients,” Eric Dufresne explains. He is the Yale University John J. Lee Assistant Professor of Mechanical Engineering, and also the one who developed the holographic optical tweezers technology, in the late 1990s. The method is used to manipulate small particles using just light. The expert is also a co-leader of the research effort, alongside Yale School of Engineering & Applied Science Postdoctoral Associate Holger Kress.
In the new approach, a class of sponge-like microparticles is used to gradually release the chemicals that neutrophils follow. The motion of the particles is directed via beams of light, which allows researchers to control their motions in three dimensions. “Understanding how cells move in response to chemical stimuli can help us better understand how a single egg develops into a complex organism or how brain cells grow into a network of neurons in a growing embryo, or how cancer cells spread through the body. This technique could give biologists insight into the ways many different types of cells respond to environmental stimuli in a wide range of situations,” Kress concludes.
 14 DAY TRIAL //
14 DAY TRIAL //