One of the primary limitations of lithium batteries has thus far been their inability to power electric cars for long distances. But that is about to change, since experts at the Stanford University announce the creation of a new type of lithium battery, that features a new cathode material.
The component is made up of graphene cages, in which researchers were able to trap sulfur particles. This combination is apparently exquisitely suited for applications in electric vehicles, experts say.
Lithium batteries are a staple of modern society, as they are used to power up phones, mp3 players, laptops, and nearly any other type of portable device you can think of. But sizing up their use, to electric cars for example, has thus far proven technically challenging.
What car batteries need to be able to do is carry large amounts of electricity, and release it as quick as a hungry engine demands. The devices also need to be capable of doing this over and over again.
Experts have known for a long time that improving the cathode's specific capacity is the way to move forward, but progress in this area has been slow. Achieving a decent energy efficiency as well as a good cycle life has proven more complex than originally thought.
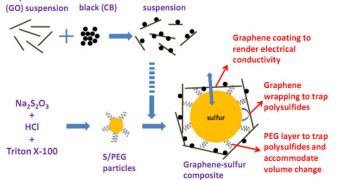
But Stanford expert Hailiang Wang and his group managed to create a new lithium battery that uses sulfur trapped in graphene as a material for the cathode. Graphene is a hexagonal, 2D carbon compound, and also the toughest material ever obtained.
It boasts some remarkable physical and chemical properties, though investigators are still a long way from realizing its true potential. The electrical properties this material has make it a prime choice to become the official replacement for silicon in all electronic devices.
The team used graphene in combination with sulfur, which has great potential for storing electricity, but is an extremely poor conductor. During each charging cycle, sulfur is also known to swell during discharges, which causes the element to crumble.
But the team used a few tricks to avoid this problem. First, they coated the nanoscale sulfur particles with a plastic-like compound called polyethyleneglycol (PEG), and then they encased the structures in graphene cages.
The interactions between sulfur and carbon rendered the former electrically-conductive, allowing it to release its impressive electrical charge according to needs, Technology Review reports.
“It is worth noting that the graphene-sulfur composite could be coupled with silicon based anode materials for rechargeable batteries with significantly higher energy density than currently possible,” Wang concludes.
 14 DAY TRIAL //
14 DAY TRIAL //