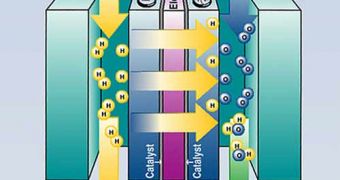
A fuel cell is an electrochemical energy conversion device. It produces electricity from external supplies of fuel (on the anode side) and oxidant (on the cathode side). These react in the presence of an electrolyte. Generally, the reactants flow in and reaction products flow out while the electrolyte remains in the cell. Fuel cells can operate virtually continuously as long as the necessary flows are maintained.
Now, a new type of solid-oxide fuel cell promises to become the next generation electricity generators. Built by Acumentrics Corporation, it has increased the output of a single fuel cell tube from 1 watt to 60 watts.
The fuel cell runs on natural gas, propane, ethanol, diesel, biogas and biodiesel, and although using non-hydrogen fuel means that the cell will produce CO2, they were designed to consume half as much fuel as a comparable small-engine generator, per kW.
This means this next generation fuel cells can produce the same amount of electricity, while consuming half as much fuel and producing half as much CO2. The eco-friendly fuel cell can be set up to simultaneously create electricity and convert noxious gases such as methane into carbon dioxide and water that are heated up to 800 degrees C (1472 F) from the controlled combustion of oxygen.
Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, rural locations and in certain military applications. A fuel cell system running on hydrogen can be compact, lightweight and has no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability.
At last, some good news for the environment! We can only hope that more environmental friendly applications emerge, for the benefit of all mankind.
 14 DAY TRIAL //
14 DAY TRIAL //