A team of investigators at the University of Pennsylvania recently determined how the “coffee ring effect” is produced on surfaces. The new discovery could enable the creation of new coating materials, as well as advanced inks and various types of novel paints.
The coffee ring effect is a termed coined to describe the odd way in which color appears to gravitate towards the edges of the rings coffee mugs leave behind on surfaces. This can be seen on tables, kitchen cloths, clothes and small dishes.
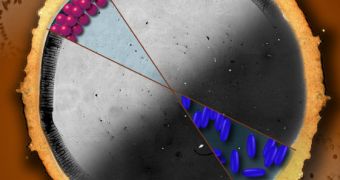
Apparently, this type of behavior is dictated by the nature of the particles that are suspended in the mix. In other words, other types of particles – such as for instances in other types of drinks – may not produce the same effect. In either case, coffee does, hence the name.
Details of the new investigation were detailed in the August 18 issue of the top scientific journal Nature. The UP research team that led the investigation was coordinated by expert Arjun Yodh.
“We found that if you change the shape of the particles in the solution, the coffee ring effect goes away, and you end up with a uniform coating,” says UP graduate student Peter Yunker, who was also a member of the research team.
According to fluid dynamics, coffee rings form because fluids tend to flow outwards the edges of a drop of liquid, before it dries up. This force is what gives coffee drops their ring-like appearance in the end, but not all fluids exhibit the same type of chemical reactions while drying up.
Milk, for instance, has the tendency to exert a high level of surface tension on cereals, making them clump together at the middle of a bowl, rather than on the edges. On the other hand, water may form raised droplets because H2O molecules are more likely to interact with each other than with air.
“If you make the particles elongated or ellipsoidal, they deform the air-water interface, which causes the particles to strongly attract one another,” Yunker explains. What the study implies is that elongated particles behave differently than round ones in a liquid.
“This work gives us a new idea about how to make a uniform coating, relatively simply. If you change the particle shape, you can change the way a particle is deposited. You can also make mixtures,” Yodh explains.
“In some cases, even just a small amount of ellipsoids can change the way the particles deposit when they dry,” the investigator concludes. The new study was sponsored by the US National Science Foundation (NSF).
 14 DAY TRIAL //
14 DAY TRIAL //