A group of investigators at the Brown University, in Providence, Rhode Island, argues in a new study that boron atoms can be used in particular configurations to produce a 2D material similar to graphene.
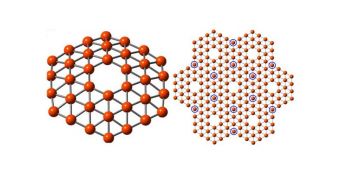
The team says that the nanomaterial would be made up of a unique arrangement of 36 boron atoms, arranged in a flat disc, and featuring a hexagon-shaped hole in the middle. This counterpart to graphene has been proven possible by supercomputer simulations conducted at the university.
Graphene is a 2D material featuring carbon atoms arranged in a honeycomb-like, hexagonal pattern. It boasts tremendous physical and chemical properties, and has generated over 4,000 research papers since first discovered at the University of Manchester, back in 2004.
Brown researchers say that the new material, tentatively dubbed borophene, could soon be added to the 2D materials class. Details of how the material should look like, and predictions about its properties, appear in a paper published in the latest issue of the top scientific journal Nature Communications.
This interest in the capabilities of boron stems from the fact that this element neighbors carbon in the periodic table. Though the Brown team has proven that 2D boron sheets can exist, the work suggests that this is only possible when the atoms are arranged in very specific configurations.
Due to the fact that it has one fewer electron than carbon, boron cannot be arranged in a hexagonal lattice similar to that of graphene. Simulations conducted in the new study revealed that the atoms need to be arranged in triangular patterns, with hexagon-shaped holes scattered throughout the lattice.
“That was the prediction, but nobody had made anything to show that’s the case,” says Brown University professor of chemistry Lai-Sheng Wang, who led the university research group. Wang has been studying the possibilities of boron-based chemistry for many years.
The expert says that his group at Brown was recently able to produce that first experimental proof that the material they are proposing is actually possible. The team managed to put together a cluster of 36 boron atoms in a triangle configuration, featuring a perfect hexagon hole right in the middle.
“It’s beautiful. It has exact hexagonal symmetry with the hexagonal hole we were looking for. The hole is of real significance here. It suggests that this theoretical calculation about a boron planar structure might be right,” Wang says.
The expert says that future borophene sheets could be made out of multiple 36-atom clusters tied together. The exact nature and requirements of the manufacturing process have yet to be fully predicted and understood, but the Brown team is hopeful that graphene will soon have a companion in its class.
 14 DAY TRIAL //
14 DAY TRIAL //