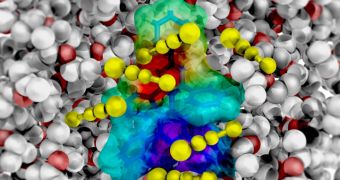
Whenever chemicals combine and react inside a solution, the outcome of their interactions is dictated by the molecular “spectators” that are closest to the site of the action. This layer has now been finally observed.
Chemists have known about the existence of the first solvation shell for decades, but limited technological means have thus far prevented it from being observed directly.
The shell can sense and dictate the fate of nearly every chemical reaction, and yet it was until recently one of the greatest unknowns in the field of chemistry.
The feat was achieved at the University of Michigan, by a team of scientists led by researchers Kevin Kubarych and Carlos Baiz.
The group published its results online, in the August 25 issue of the esteemed scientific Journal of the American Chemical Society (JACS).
One of the main reasons why the solvent shell could not be observed in action was the fact that the speed of the chemical reaction itself was too high. Another was the difficulty in identifying which solvent molecules lied in the first solvation shells.
Experts explain the differences between these molecules as if the small structures were spectators in a show – the team needed only those in the front row, not in the second and third.
Identifying the first solvation shell is therefore reliant on ultrafast time resolution, the ability to initiate the reaction, and also to track the response of the solvent shell.
The new analysis method relies on identifying changing resonance frequencies of the surrounding molecules, in chemical solutions.
“This level of detailed information on the complex environments common in chemical transformations is unique, and promises to offer remarkable insight into the understanding of natural and artificial charge transfer reactions – processes that are of fundamental importance in contexts ranging from cellular respiration to solar energy conversion,” Kubarych explains.
The new investigation was sponsored by the UM Rackham Graduate School, and also by the US National Science Foundation (NSF).
The researchers conclude by saying that the changes in resonance frequencies allows their new analysis tool to identify and keep up with the ultra-fast events taking place in the chemical reactions that are analyzed.
 14 DAY TRIAL //
14 DAY TRIAL //