Georgia Institute of Technology (GIT) and the National Institute of Standards and Technology (NIST) experts have announced that they managed to determine the energy spectrum of graphene, the “wonder” carbon compound that has only two dimensions. By using complex measurement techniques, they succeeded in determining the peculiar energy spectrum that graphene had, which may soon lead to exciting new applications for the outstanding material.
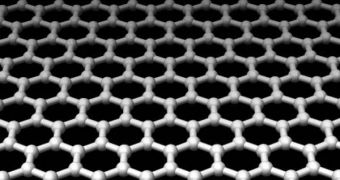
Graphene is a relatively new material, discovered only in 2004 by a team of experts at the University of Manchester, in the UK. It features a honeycomb-like structure, with atoms arranged in ever-repeating hexagonal structures. However, its main feature is that it's only one-atom-thick, which means that it has the potential to be used for anything from creating transparent semiconductors to devising the next-generation of super-fast and reliable computer chips.
One of the main reasons why graphene has become so widely popular with the international scientific community is the fact that it directs electricity very efficiently. In addition, electrons inside this material are about 100 times more mobile than those in silicon, even if they are both at room temperature. Basically, at least in theory, graphene-based electronics could exceed the performances of the best silicon-based processors hands down, under any circumstances.
But physicists are mostly amazed by one very small thing – the fact that electrons and other charge carriers inside graphene seem to behave as if they had no mass to speak of. This is, obviously, impossible, at least in theory, but that doesn't stop the material from displaying the properties. By using a specialized NIST zooming instrument, capable of observing the graphene layer at about a billion times the original magnification, the GIT/NIST team managed to observe that the “weightless” electrons behaved very differently from regular ones when subjected to a magnetic field.
Rather than starting to be moved in circular orbits, at predefined and equally distant energy layers, the electrons in graphene exhibited a very strange and chaotic functioning pattern, which the researchers said was caused by their seeming weightlessness. Also, because they stack in different orientations, graphene layers have only weak atomic links between them, and can easily slide on top of each other. The find could aid experts in discovering new methods of producing large amounts of the element.
 14 DAY TRIAL //
14 DAY TRIAL //