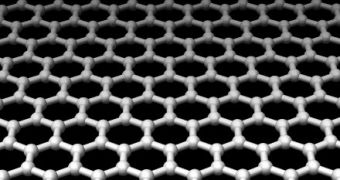
Graphene has a thermal conductivity fifty percent higher than that of carbon nanotube, and about 10 times higher than metals such as copper and aluminum. Better still, graphene combines both semiconductor and metal properties which make it ideal for a replacement for semiconductors currently used in computer chips. Graphenes are basically single-atom-thick sheets of graphite material, or unrolled carbon nanotubes if you may.
This means that graphenes, having fewer atoms and better thermal conductivity than many other common metals, are practically faster at conducting electrons, say physicists from the University of Maryland. The study conducted by Michael Fuhrer reveals for the first time that thermal vibrations on the conduction of electrons in grapheme have smaller effects on the electrons in graphene, thus allowing them to travel at higher speeds.
Past experiences with material tought us that with greater amount of energy inputed through heat, the atoms inside the material vibrate faster and faster, thus increasing the electrical resistance. This means that in order to minimize the effect of temperature on the electrical conductivity, the material needs to be cooled to temperatures close to absolute zero. At absolute zero, the electrons inside a material should theoretically stop moving, hence giving it zero electrical resistivity - superconductivity. Unfortunately, absolute zero cannot be achieved practically.
Graphene, on the other hand, experiences an electrical resistivity of about 1.0 microOhm-centimeter, meaning 35 percent less than that of copper - which is the lowest-resistivity material at room temperature. "Other extrinsic sources in today's fairly dirty graphene samples add some extra resistivity to graphene, so the overall resistivity isn't quite as low as coppera at room temperature - yet," said Fuhrer. Albeit graphene must exploit one of its best properties in relation to other material, the extremely low number of electrons.
Let's take semiconductors for example. Silicon has an electron mobility of 1,400 square centimeters per Volt-second, while indium antimonide experiences 77,000 square centimeters per Volt-second. Graphene has an electron mobility of 200,000 square centimeters per Volt-second, more than twice of that of the highest mobility conventional semiconductor.
"Interestingly, in semiconducting carbon nanotubes, which may be thought of as graphene rolled into a cylinder, we've shown that the mobility at room temperature is over 100,000 cm2/Vs," said Fuhrer. By using graphene to create electronic devices such as transistors, we could basically triple the frequency of microprocessors overnight.
Additionally, graphene's high electron mobility translates into higher conductivity, which may have applications in chemical and bio-chemical sensing devices. Low-resistivity, mechanically tough, high-conductivity, transparent graphene films could also have applications in the touch screens and photovoltaic cells.
Although it has been shown that graphene has an electron mobility of up to 200,000 cm2/Vs, the testing rig only allows it to experience about 10,000 cm2/Vs, due to the fact that the one-atom-thick material must sit on a substrate of silicon dioxide, which greatly reduces the electron mobility, as electron vibration is directly transfered from the substrate.
The effect is known as "remote interfacial phonon scattering" and represents only a small correction to the mobility in a silicon transistor, however because graphene is inefficient at scattering electrons, the effect becomes important in graphene.
 14 DAY TRIAL //
14 DAY TRIAL //