In a groundbreaking, new study conducted at the Max Planck Institute of Colloids and Interfaces, German researchers have set the basis for understanding how nanoparticles appear in nature. The work has also yielded cadmium sulphide particles coated in membrane bubbles at the microscopic scale, to be used as “position lights” in cellular proteins, or in display screens. The innovation could also benefit the field of optical computing, once the finds are further refined. The researchers based their new material on two new instruction manuals, devised to help scientists create nanoscale light bulbs.
Cells inside living organisms are arguably the most efficient things in nature. Like micro-scale factories, they work tirelessly to provide energy and new constructs from nanoparticles, while consuming vast amounts of matter in the process. During the course of evolution, they adapted to using certain tools for this job, such as peptides, which can shape chalk inside the cell, for instance, into any shape needed. The instructions for these actions are encoded in the genes on each chromosome.
“We used the fact that cells represent a closed reaction container as a model for the synthesis of nanoparticles,” the leader of the Max Planck Institute of Colloids and Interfaces membrane study group, Rumiana Dimova, explains. Membranes are the boundaries of the cells, being strong enough to contain the nucleus, cytoplasm, genes, proteins, and countless processes inside its perimeter, while at the same time remaining permissive enough to allow for the necessary substances to pass in and out of the cell, regulating its functions.
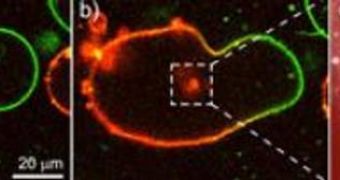
The membrane bubbles (vesicles) the team built are very similar to cellular membranes, in that they also provide a closed reaction container. They created two types of vesicles, one loaded with sodium sulphide, and the other with cadmium chloride. With the aid of a short, but extremely powerful, electric burst, the team then fused the two membranes together, allowing the reactants to merge. As a result, the non-water soluble cadmium sulphide was formed, which precipitated in the form of nanoparticles.
“Because the reactants are only present to a limited extent in the fused bubbles, the particles only grow to a size of four nanometres,” Rumiana Dimova adds. She says that her group was able to observe these reactions by marking each of the reacting substances with fluorescent molecules. Through a powerful microscope, the precipitation of nanoparticles became visible, as fluorescent light illuminated the eerie scene. “With our method, we succeeded for the first time in producing particles with a certain diameter in vesicles whose size corresponds to that of cells,” the expert concludes.
For the first time ever, in the new study, researchers proved that the addition of peptides was not absolutely necessary for obtaining this type of reactions. Previously, other study groups hypothesized that they were indispensable to the process.
 14 DAY TRIAL //
14 DAY TRIAL //