A new artificial scaffold structure could in the near future provide support for cardiac muscle cells to be grown in the lab. The innovation could save the lives of millions in the developed world.
Congestive heart failure is the most common cause of death in Western countries, and the condition most likely develops as the result of people suffering heart attacks. The event is no longer fatal in itself, but its consequences definitely are.
These results could be addressed if researchers would be able to construct new cardiac muscle tissue that would exhibit the modifications heart attacks usually trigger. This is now possible thanks to the scaffolds developed at the University of Washington.
The innovative equipment can host stem cells that are allowed to develop into cardiac muscle cells alone. The scaffold was also found to encourage the creation of blood vessels in living animals.
“Today, if you have a heart attack there's nothing that doctors can do to repair the damage. You are, in essence, sentenced to a downhill slide, developing congestive heart failure that greatly shortens your lifespan,” UW professor of bioengineering Buddy Ratner says.
“Your body can't make new heart cells, but what if we can deliver vital new cells in that damaged portion of the heart?” Ratner, who is also the author of the new investigation, adds. The research team published details of its accomplishment in the latest issue of the esteemed journal Proceedings of the National Academy of Sciences (PNAS).
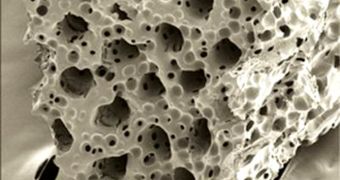
The new scaffold is tiny, tubular, and porous. Its main job is to stabilize and support cardiac cells while they are still in their fragile state. The material can be injected into the damaged heart, promoting cell growth directly at the target location.
As soon as the scaffold completes its duty, and allows for the cardiac tissue to develop, it melts away, and leaves the body without causing any permanent side-effects.
“We're very optimistic that this scaffold will help keep the muscle cells alive after implantation and will help transition them to working heart muscles,” explains UW professor of pathology and bioengineering Chuck Murry, a coauthor of the PNAS paper.
“The first thing that transplanted heart cells have to do is survive. And when you transition them from a culture dish to the body, initially they don't have a blood supply. So we have to promote the host blood supply as fast as possible,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //