A University of Bristol spin-off company called Azellon Cell Therapeutics Ltd. has now collected enough funding to begin human clinical trials of an innovative stem cell bandage it developed. The tool can be used to treat torn meniscal cartilages, and is the first of its kind
Sun Capital partner Hugh Osmond just awarded the company £0.65 million ($1.03 million or €758,193), bringing the total amount of funds Azellon put together to £2.25 million ($3.58 million or €2.62 million).
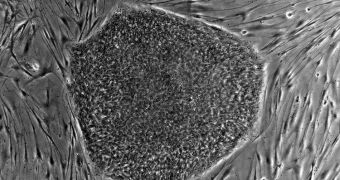
The bandage the company wants to test is made up of adult, autologous stem cells. This means that the stem cells it uses are adult ones, collected straight from the patient. This approach reduces the possibility of implant rejection to nearly zero, promising excellent results in the long-run.
Thanks to the new investment, the UB spin-off is now able to start human clinical trials, in order to assess how the treatment goes in real-life situations. If the tests are successful, then we could expect to see the technique applied in hospitals around the world over the next few years.
According to officials at the university, the company has also just received approval for its Cell Bandage product trials from the UK Medicines and Healthcare products Regulatory Agency (MHRA).
At this time, the only viable treatment for torn meniscal cartilages is the surgical removal of the entire meniscus, a procedure called meniscectomy. Bristol investigators estimate that around 1.7 million people around the world undergo the procedure yearly.
There are numerous drawbacks to removing the entire meniscus, such as for example the fact that the procedure carries a high risk of triggering the early onset of osteoarthritis. Further along the line, the procedure leads to the need for total knee replacement surgery.
Thus far, Cell Bandage has only been tested in cell cultures, but the approach showed great promise. Human clinical trials are the next logical step, scientists say, but starting them proved problematic, especially due to funding shortages.
“With permission for a trial from MHRA and completion of this funding round, we are now ready to get going on our safety trial; it’s an important moment for Azellon and for stem cell research,” UB School of Cellular and Molecular Medicine head, professor Anthony Hollander, explains.
He is also the Chief Scientific Officer of Azellon Cell Therapeutics Ltd. The expert says that the clinical trials will being in May 2012, and will be conducted at the Southmead Hospital, in Bristol.
 14 DAY TRIAL //
14 DAY TRIAL //