Wear OS smartwatches could soon get support for ECG, the feature that Apple introduced last year on the Apple Watch Series 4.
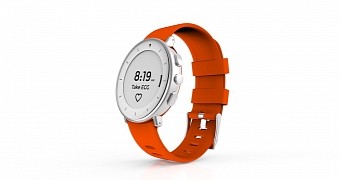
Verily, a company backed by Google, announced this week that its Study Watch is labeled as a Class II medical device after receiving FDA approval for ECG readings.
In other words, its limited watch, which is only offered to customers based on a prescription, offers electrocardiogram support as well, being able to detect a series of heart diseases like atrial fibrillation.
“The FDA-cleared Study Watch is a prescription-only device intended to record, store, transfer and display single-channel ECG rhythms, and is indicated for use by healthcare professionals, adult patients with known or suspected heart conditions and health conscious individuals. The ability to take an on-demand, single-lead ECG, can support both population-based research and an individual’s clinical care,” the company said.
Google’s Pixel Watch
While at first glance this doesn’t mean anything for the Wear OS world, the Study Watch receiving FDA approval is huge news for the Wear OS device ecosystem.
With Verily being part of Alphabet, Google is expected to become more committed to such features on devices running its wearable operating system, so the company may speed up the development of ECG support on Wear OS. Furthermore, Google has recently purchased key smartwatch assets from Fossil, and this could be a sign that the company may even explore building its own smartwatch.
If this is the case, today’s announcement is even more important, as it could pave the way for Google bringing ECG support on its device.
For the time being, however, the Apple Watch Series 4 remains the only device broadly available to customers with such capabilities. By the looks of things, it won’t take too long until more devices bundle ECG features, so expect more news on this front shortly.
 14 DAY TRIAL //
14 DAY TRIAL //