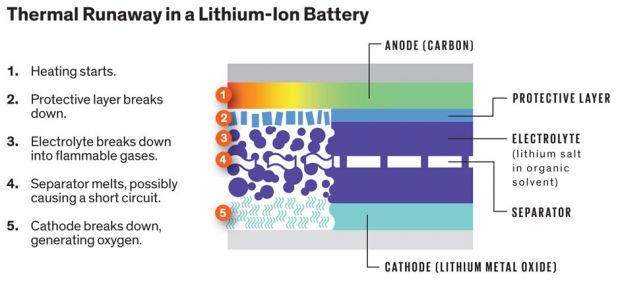
Li-ion battery can explode. Yes, and they will do so only in certain conditions. When lithium-ion batteries overheat, they will burn through its internal segments and at times even explode.
Now you could quite easily be carrying around three or four thousand milliamp hours in your pocket. That’s a lot of energy to be released if something goes wrong, in your pocket.
The explosion is usually called Thermal Runaway, and it happens when the battery is overcharged repeatedly or physically damaged. The barriers between the halves are ruined and can cause an exothermic reaction.
Usually, this happens when layers of dendrites, deposits of lithium metal can pierce the barrier between electrodes. If this build up increases, the battery will lose capacity and can reach TR.
You're safe, people
Stanford University latest studies suggested that two additives, lithium nitrate and lithium polysulfide can act as battery boosters and dendrite-busting solution that protects electrodes. Instead of dendrites, lithium deposits are formed causing no damage to the battery.
Experiments conducted by Stanford University showed that after 300 discharge cycles, the doped batteries still had 99% efficiency, whereas a battery doped with only lithium nitrate was down to 92% after just 180 cycles.
This new development will continue on lithium-air and lithium-sulfur rechargeable batteries, which are susceptible to dendrite formation. These batteries will be capable of storing up to 10 times more energy per unit mass than present day batteries. Experiments suggest that metal-made batteries like magnesium, calcium, or aluminum could also be affected by this research.
 14 DAY TRIAL //
14 DAY TRIAL //