Presently, countries in West Africa are battling a major Ebola outbreak. Looking to contain the spread of this deadly disease, the World Health Organization has agreed to give the green light to clinical trials intended to determine the efficiency of drugs designed to either prevent or fight infection.
The Organization explains that, for the time being, there is no Ebola drug or vaccine that has been proven to be both safe and effective in humans. However, several treatments are in the works, Nature informs.
It is these treatments that the World Health Organization wants scientists to test during the planned clinical trials. The end goal is to determine whether or not any of them could help contain the ongoing Ebola disease epidemic in West Africa.
Clinical trials for new drugs are usually carried out in state-of-the-art medical facilities and involve healthy volunteers. However, specialists with the Organization think that, given the severity of the Ebola outbreak, it's OK to make an exception this time.
“Although the slow regulatory route for testing drugs and vaccines is ill-adapted to the rapid response needed during an outbreak, it is an obstacle that must be overcome. We don’t have the luxury of being able to test these in humans outside of an epidemic setting,” said tropical-disease specialist Jeremy Farrar.
Information shared with the public says that a team of experts are scheduled to meet in Geneva, Switzerland, this coming September 4 – 5. During this meeting, the scientists are to discuss which drugs and vaccines should be included in the clinical trials.
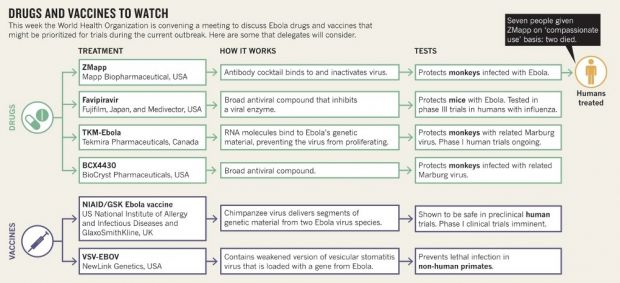
The infographic below documents some of the drugs and vaccines the World Health Organization and specialists working closely with it are to focus on. Of these drugs, one dubbed ZMapp has proven surprisingly effective in terms of treating Ebola in monkeys and has even been tested on a few human patients.
Once the scientists attending this meeting in Geneva settle on the drugs and vaccines that have until now shown enough promise to be suitable for clinical trials, small groups of people infected with Ebola will receive one treatment or another. The drugs and vaccines will be administered in an organized manner, and people's responses to them will be thoroughly documented.
Since Ebola has a high mortality rate, i.e. it kills 53% of the people it comes to infect, specialists say that, should either one of the drugs included in these clinical trials be successful, there is little doubt that its effectiveness will almost immediately be recorded.
 14 DAY TRIAL //
14 DAY TRIAL //