Although virtually everything in the Universe is made of plasma, this state of the matter remained unknown until the 20th century. The reason is that in order to understand plasma, you need to understand what atoms and molecules are made of. Plasma is a state of matter that contains not only atoms and molecules but also their components in a free state: electrons and ions free to fly around. Most plasma is ionized gas (like the one found in outer space, in stars, in the ionosphere, in flames or in fluorescent tubes, to name just a few places). In neutron stars one also has liquid plasma and, at their surface, even solid plasma. Physicists also speculate that another form of plasma, called a quark-gluon plasma, might have existed short after the beginning of the Universe, but insofar the experiments designed to test these theories have been inconclusive.
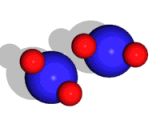
In this image, you can see a representation of a water molecule. There are two hydrogen atoms and one oxygen atom; the hydrogen atom is shown to have one proton and one electron, and the proton is made of three quarks; the oxygen atom has neutrons in its nucleus alongside its protons.
An atom or a molecule has an equal amount of electrons and protons and thus, it is electrically neutral. Because of this, molecules (or atoms) cannot interact with each other (there are no forces between them) unless they get very close. When the molecules are very close to each other, slight variations in the distribution of the electric charge inside them begin to matter.
For example, the water molecule is slightly more negatively charged toward the oxygen atom and slightly more positively charged toward the hydrogen atoms (the oxygen is larger than the hydrogen atoms and thus exerts a stronger attraction to the hydrogens' electrons leaving the two protons somewhat "exposed"). Because of this, when water molecules are close to each other they can interact - the oxygen side of one molecule (slightly negative) attracts the hydrogen side of some other molecule (which is slightly positive). (This type of interaction is called a "hydrogen bond".)
The major difference that appears in case of plasma is that, because of the presence of electrically charged particles (electrons and ions), there are electric forces on a larger scale and, because the charges are in motion, magnetic fields. This difference leads to a much more complicated behavior of the entire bulk material.
Although plasma is made of electrically charged particles, it has one important characteristic: it is electrically neutral overall. For example, the Sun as a whole is an electrically neutral body although it is composed of free protons and electrons and, consequently, it is ravaged by large scale magnetic fields. However, a beam of electrons like the one in a TV tube is not considered plasma.
The maximum distance at which one can observe a departure from the overall neutrality is called Debye length. For example, the intergalactic plasma has a Debye length of 10 km, the Earth's ionosphere of 1 mm, and a fluorescent tube of a tenth of mm. The importance of this distance is that it can be demonstrated that a charge is affected only by the electric forces coming from inside the bubble of this radius. In other words, although the plasma is filled with electric charges they don't have long range electric effects - the mobility of the charges leads to a screening effect. This is the reason, for instance, why we don't have large electric forces coming from the cosmic plasma in the other corner of the galaxy. (However, there might be magnetic forces. But because magnetic forces also depend on the velocity of the moving charges, in order to have a significant galactic-scale magnetic force the free intrestellar protons should move at enormous speeds - close to the speed of light. One theory of dark matter, the matter invented to explain the curious and otherwise mysterious rotation of galaxies, claims indeed that dark matter is made of "relativistic protons", protons moving at very high speeds.)
There are other forms of matter that resemble plasma but which are not usually considered as such. They include ionic solutions, such as salt-water or the "plasma" in blood, and metals. The liquid solutions have ionized molecules, but no free electrons; metals (and some doped semiconductors) have free electrons but no free ions. (Although ionic liquid solutions are not normally considered plasma the same Debye length reasoning applies to them too.)
Where does plasma come from?
In outer space, there are free protons and electrons that formed after the Big Bang and that may have never been a part of any atom. Much of the interstellar and intergalactic gas may be made of such basic plasma. However, once atoms and molecules have formed, an input of energy is necessary for breaking them apart. This input of energy can come from various sources and thus, there are many ways by which plasma can be formed.
Inside stars it is gravitation that pushes atoms together and smashes them so hard that they cannot remain intact. Thus, there is a sea of atomic nuclei, especially protons (hydrogen nuclei), and electrons. Inside stars gravitation is so powerful that it fuses protons together and produces helium nuclei (made of two protons and one or two neutrons), light and neutrinos. (Read more about stars here and here.)
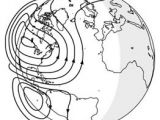
In case of the Earth's ionosphere, the region of the atmosphere at heights of above 80 km (50 miles), the gas is ionized by the ultraviolet light coming from the Sun. The image shows the electric currents that appear when the Sun shines on Earth.
The Debye bubbles in the ionosphere are small, only of the order of 1 mm, but nonetheless, they are sufficient to influence radio waves. The first radio communication over large distances used the fact that radio waves are reflected by the ionosphere - as well as by the surface of the Earth (or by the ocean). Thus, using this multiple reflection and a sufficiently powerful emitter one can send a radio signal to another part of the globe. The ionosphere also reflects back into space the radio waves coming from outside the Earth, making it difficult to observe the sky in the radio domain.
In case of fire there is a chemical reaction: oxygen reacts violently with the material (the fuel) and produces heat. When the same oxidation reaction goes more slowly, for example in case of rusting, the release of energy via heat is much slower (i.e. the power is smaller) and we don't witness fire.
Fire happens because the heat generated by the oxidation reaction makes the air molecules agitate very vigorously. The molecules bump into one another and during this process their motion energy (kinetic energy) is transferred temporarily to their electrons. The electrons jump further away from the atomic nuclei, but most of them don't go sufficiently far away to actually escape their grip (this process is called "excitation"). When the electrons return to their original orbits, they release the extra energy as light. Some of the electrons might escape the molecules, leaving behind charged ions, but they are quickly caught back by other ions. Fire is thus only a very limited form of plasma. The image shows different flames caused by different oxygen supplies.
Inside a fluorescent tube, there are electrons ejected from the negatively charged electrode (called cathode). The electrons hit the molecules of gas inside the tube and break them apart. As a consequence, there is a chain reaction, one initial electron producing a shower of subsequent electrons. However, these electrons are less and less energetic and at a certain distance from the electrode they are unable to further ionize the gas molecules. The electrons then just "excite" the molecules, and when the molecules' electrons return to their stable orbits they emit light.
The ions produced by the electron shower are positively charged (because the molecules have lost one or more electrons) and thus, are attracted by the cathode. As they accelerate toward the cathode, they also bump into other molecules exciting or even ionizing them. As you can realize, the processes going on in such a tube are quite complex, with different things happening in different portions of the tube. This complexity is revealed even by the looks of the discharge. Try to explain, for example, why that maximum of intensity appears where it does or how it is influenced by a change in the applied voltage.
Commercial fluorescent tubes, used either for illumination or as "black light" in clubs, are coated with a fluorescent substance. This substance absorbs the light emitted inside the tube and emits light on other frequencies. This is useful both for assuring that the tube emits the desired color of light (white light or ultraviolet light - the so-called "black light") and for increasing its power efficiency: in case of illumination tubes the coating absorbs the ultraviolet light (which is invisible) and releases that energy as visible light or, conversely, in case of club "black lights" it releases all the light as ultraviolet light (which makes various materials shine in unusual ways).
Why is plasma so interesting?
What makes plasma a very intriguing form of matter is that it is even more basic than the gas, in the sense that the gas is more ordered, but in the same time, it exhibits a highly complex behavior. This image of a solar eruption compared to the size of our planet should give you a vivid impression of what plasma means.
But this complex behavior is very basic - it is prior to the appearance of any complex structure. When one gets from plasma to a gas, one is witnessing particles like protons, neutrons and electrons organizing into structures like atoms and molecules. Furthermore, when we get from gas to liquid and then to solid we observe atoms and molecules organizing into a large structure - the crystal. The intermediary step between gas and solid, the liquid phase, also exhibits very complex behaviors, much more complex than one can see in case of gases or solids. (Read for example about water.)
These complex behaviors, both in case of plasma and in case of liquids, are the consequence of what are known as "collective phenomena" - the situation in which each particle interacts simultaneously with many others in constantly changing ways. In case of plasma, the particles are the elementary (electrons) or almost elementary (protons and neutrons) particles. In case of liquids, the particles are the atoms and molecules.
It is interesting to note that the complex behavior is not necessarily a consequence of a complex structure and that a more complex structure can sometimes exhibit a much simpler behavior.
 14 DAY TRIAL //
14 DAY TRIAL //